Figure 3.
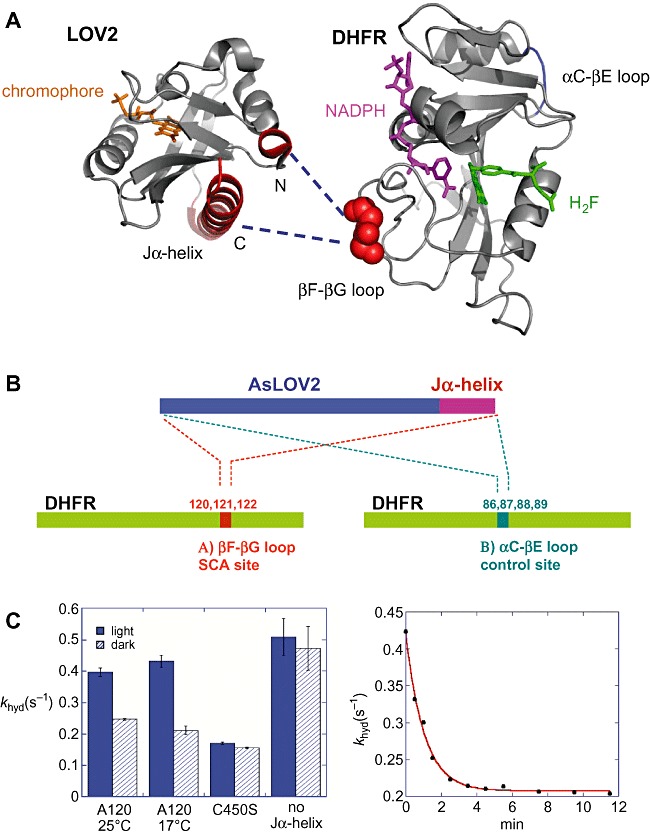
A. Structures of AsLOV2 (pdb entry: 2v0u) and DHFR (pdb entry: 1rx2), illustrating surface‐exposed SCA sites (red), the chromophore FMN (orange), NADPH (magenta) and folate (green). The SCA method identifies networks of statistically correlated amino acid residues in the LOV domain as well as in DHFR. The network links the FMN binding pocket of the LOV domain to N‐ and C‐terminal helices (shown in red) that undergo significant conformational changes upon light activation (see Fig. 3). The design strategy was to insert the light‐sensing domain into one of the surface positions (βF–βG loop, in red) in the DHFR network so that the light signal can be transmitted to the DHFR active site though the network. B. Schematic representation of the LOV2–DHFR chimeric constructs. To functionally couple the light‐induced signal with the DHFR catalysis, sets of chimeric proteins were generated. Site A (amino acids 120–122) is an allosteric site within the coupled network of DHFR, while site B (amino acids 86–89) is a control site that is not correlated with the active site. C. Light‐dependent enzyme activity in the LOV2–DHFR chimera. Hydride transfer rates (khyd) were measured by a stopped‐flow kinetic method under dark (shown as hatched bars) and light‐exposed conditions (blue bars). Among the chimeras, light dependence was observed only when the LOV domain is attached to a specific position (A120). This chimera exhibited a twofold increase of hydride transfer rate in the light condition. The construct with a point mutation (C450S) that locks the LOV domain into the dark state or a chimera lacking the Jα‐helix did not show the light dependence. The kinetics of the dark recovery of the hydride transfer rate exhibits a single exponential decay (right part of C), which is comparable to the kinetics of the FMN‐thiol covalent adduct decay.
