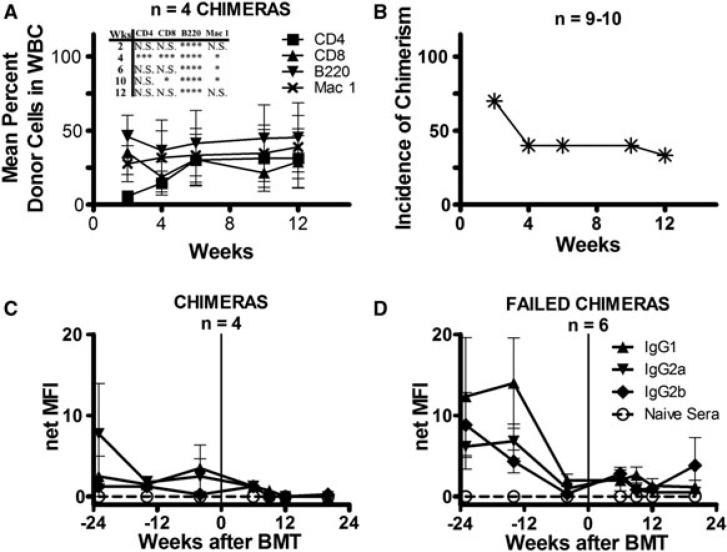
Figure 5. Chimerism and detection of alloantibody in presensitized wild-type B6 mice.
(A) Mean percent repopulation of donor cells in each WBC lineage in presensitized wild-type (WT) B6 mice over time as measured by flow cytometry. All mice received anti-CD8, anti-CD4, anti-NK1.1, anti-CD40L and anti-OX40L along with 3 Gy TBI and 80 × 106 B10.A BMCs. Only mice that maintained chimerism are shown. Also shown are statistics comparing the percent chimerism for the CD4, CD8, B220 and Mac-1 cell lineages between the presensitized WT B6 and μMT B6 chimeras (Figure 2A) receiving the same conditioning (*0.01 ≤ p < 0.05; ** 0.001 ≤ p < 0.01; ***p < 0.001; ****p < 0.0001). (B) Incidence of chimerism over time in 10 presensitized WT B6 mice receiving the above regimen. At week 12, the incidence of chimerism among nine mice is shown because of the death of one mouse in this experiment. (C, D) Sera from WT B6 mice presensitized with B10.A skin grafts were added to splenocytes of the sensitizing strain. FITC-conjugated secondary antibodies directed against various IgG subclasses were then added to detect alloantibody recognizing B10.A alloantigens. Shown is the net median fluorescence intensity (MFI) of alloantibody in the sera of presensitized WT B6 chimeras (C), and failed chimeras (D) over time as measured by indirect flow cytometry. The net MFI was calculated by subtracting nonspecific background staining of splenocytes incubated with the respective secondary antibodies alone. Positive control MFIs for this assay were IgG1 140, IgG2a 189 and IgG2b 331. Vertical line indicates the time of allogeneic (allo) BMT.