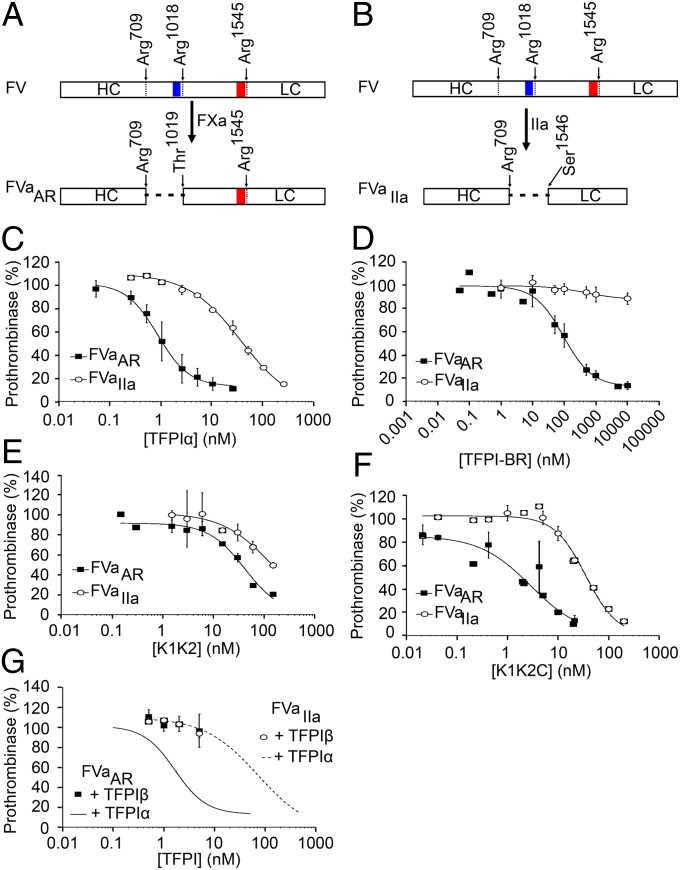
Fig. 5.
Inhibition of prothrombinase by TFPIα requires K2 and the basic C terminus. (A) FXa generated FVaAR through cleavages following Arg709 and Arg1018, leaving the acidic region of the FV B domain (red) attached to the light chain, but removing the basic region (blue). (B) Thrombin generated FVaIIa through cleavages following Arg709, Arg1018, and Arg1545, completely removing the B domain and generating the heavy and light chains. (C–G) FVaAR or FVaIIa (0.5 nM), phospholipid vesicles (20 µM), and the thrombin inhibitor DAPA (3 µM) were incubated with varying concentrations of TFPIα (C), TFPI-BR (D), K1K2 (E), K1K2C (F), or TFPIβ-expressing CHO cells (G). For G, phospholipids were omitted. Reactions were initiated by addition of prothrombin (1.4 µM) and FXa (5 nM). The initial rate of thrombin generation is reported as percent of control. All data points are shown as mean ± SD (n ≥ 3 independent experiments for each). Lines represent best-fit curves. In G, data from C are shown for reference.