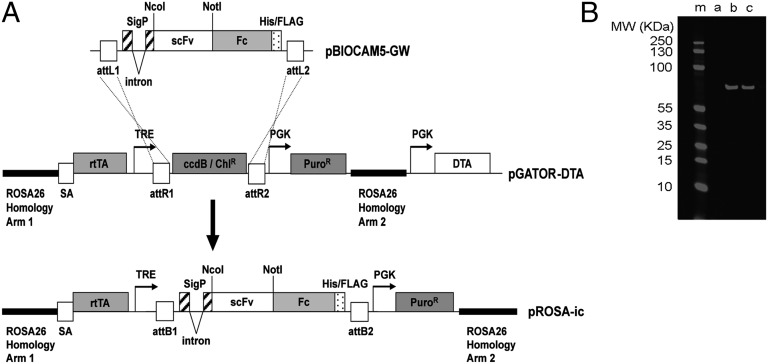
Fig. 1.
Construction of the pROSA-ic vector for targeting antibody expression cassettes into the ubiquitously expressed Rosa-26 locus of mouse ES cells. (A) Antibody genes (in the form of single-chain Fvs) were subcloned into the NcoI and NotI sites of the pBIOCAM5-GW entry vector to create a fusion with a human IgG1 Fc gene. LR Gateway recombination was used to facilitate the introduction of this scFv-Fc fusion gene into the large (14 kb) pGATOR-DTA targeting vector. The resultant pROSA-ic construct encompasses an antibody gene expression cassette within a 10-kb region of the Rosa-26 gene. This region is used to drive homologous recombination of the antibody expression cassette into the ubiquitously expressed Rosa-26 locus in mouse ES cells. The diphtheria toxin gene (DTA) is used to reduce frequency of clones with random integration of the cassette in the genome. In pROSA-ic antibody, expression is controlled by a doxycycline-responsive “Tet-On” promoter system. The final construct encodes a signal peptide directing secretion of a human scFv fused to a human IgG1 Fc domain (scFv-Fc fusion). The Fc domain drives dimerization of the resulting antibody. Abbreviations include: attL1, attL2, attR1, attR2, attB1, attB2, gateway recombination sites; sigP, signal peptide; His/FLAG, hexa-histidine Tri-FLAG peptide tag; scFv, single-chain Fv; Fc, human IgG1 Fc with C-terminal His-FLAG tag; SA, splice acceptor; rtTA, Tet-on transactivator; TRE, Tet response element; PuroR, puromycin resistance gene; DTA, diptheria toxin gene fragment A. (B) Doxycycline-inducible antibody expression in E14 ES cells. An anti-Notch antibody N1_E7 (11) was subcloned into pROSA-ic and the resultant targeting vector was used to transfect E14 ES cells. Correctly targeted puromycin-resistant colonies were selected and grown under self-renewal conditions with 0, 1, or 2 μg/mL doxycycline (lanes a–c, respectively). After 4 d expressed antibody was affinity-purified using anti-FLAG agarose. Samples were run on SDS/PAGE, blotted, and the expressed scFv-Fc was detected with an anti-FLAG mouse antibody and a secondary anti-mouse IR680-labeled antibody (Licor).