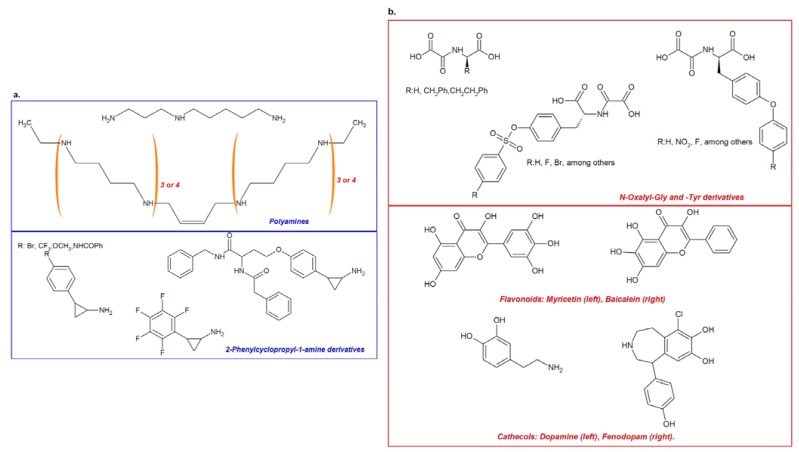
Figure 5.
Inhibitors of histone demethylation. Specific inhibitors for LSD1 can be designed according to the similarity of this demethylase’s substrates with the FAD-dependent polyamine oxidases targets (a, top frame), as can be observed for spermidin-like inhibitors, octamines (n = 3) or decamines (n = 4). Another pathway for designing amine oxidases’ inhibitors involves their mechanism of activity, targeting FAD by radical oxidation reactions with “suicide” inhibitors containing a phenylcyclopropylamine core (a, bottom frame). Histone demethylases with oxygenase activity can be inhibited by using α-ketoglutarate analogues, as these could be able to bind to Fe2+. Some of the most promising epidrugs includes some derivatives of N-oxalyl glucose and N-oxalyl tyrosine, (b, top frame), in conjuction with the recent description of flavonoids and chatecols showing competitive and non-competitive inhibition, possibly associated with these natural product’s ability to bind iron (b, bottom frame).