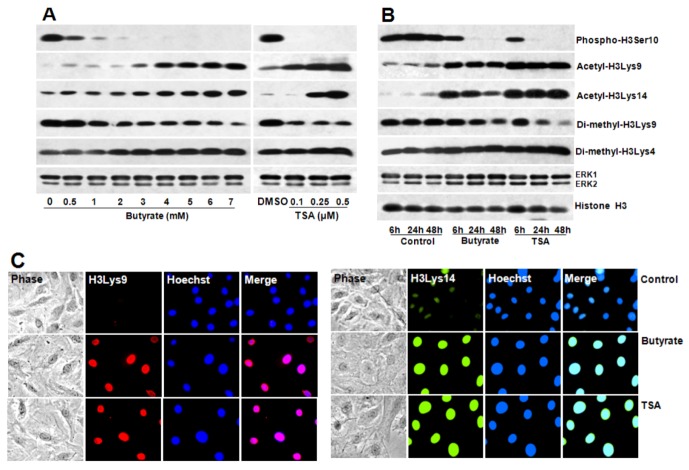
Figure 8.
Concentration and time-dependent changes in site-specific modifications of histone H3 in butyrate and TSA treated VSMC. Proliferating VSMC were treated with different concentration of butyrate or TSA for 48 h (A) or treated with 5 mM butyrate or 0.5 µM TSA for indicated periods of time (B); to determine dose- and time-dependent effects of butyrate and TSA on indicated site-specific modifications of histone H3, respectively. At the conclusion of the treatment, VSMC were processed for western analysis using appropriate antibodies to assess the effects of butyrate and TSA on histone H3 modifications. ERK1/2 was immunoblotted and used as loading control. The results shown are representative of three independent experiments. The density of each band is measured and normalized to protein loading. The data obtained are analyzed and expressed as mean ± S.D. Phospho-H3Serine10, acetyl-H3Lys9, acetyl-H3Lys14, di-methyl-H3Lys9 and di-methyl-H3Lys4 modifications stimulated by butyrate vs. control are significant to very significant (p < 0.01 to p < 0.001) depending on the concentrations of butyrate except for di-methyl-H3Lys4 modification, which is not significant at 0.5 and 1 mM butyrate concentration (A). Changes induced by TSA vs. DMSO are significant to very significant (p < 0.01 to p < 0.001) with the exception of acetyl-H3Lys14 and di-methyl-H3Lys4 at 0.1 µM TSA (A). Time-dependent changes in levels of phospho-H3Serine10, acetyl-H3Lys9, acetyl-H3Lys14, di-methyl-H3Lys9 and di-methyl-H3Lys4 histone H3 modifications are significant to very significant (p < 0.05 to p < 0.001) both in butyrate and TSA treated VSMC compared to untreated control at all periods of time with the exception of di-methyl-H3Lys9 at 6 h (B); (C) Effect of butyrate and TSA on acetylation of H3Lys9, and H3Lys14 was also determined by intracellular immunofluorescence staining of H3Lys9, and H3Lys14 followed by nuclear staining with Hoechst. VSMC treated with butyrate or TSA for 24 h were immunostained with respective antibodies and images of immunostained VSMC were captured using Nikon fluorescence microscope with a CCD camera (400× magnification).