Abstract
Background
Evidence from animal and human studies suggests that early-life stress such as physical maltreatment has long-lasting effects on the hypothalamic-pituitary-adrenal (HPA) axis and is associated with blunted HPA axis reactivity in adulthood. Few studies have investigated whether blunted HPA axis reactivity observed in children exposed to early-life stress signals social, emotional, and behavioral problems.
Methods
Participants were 190 12-year-old children (50.5% males) recruited from the Environmental Risk Longitudinal Twin Study, a nationally representative 1994 to 1995 cohort of families with twins. Cortisol responses to psychosocial stress were measured in maltreated/ bullied (n = 64) and comparison children (n = 126). We ascertained maltreatment and bullying victimization using mothers’ reports and assessed children’s social, emotional, and behavioral problems at ages 5 and 12 using mothers’ and teachers’ reports.
Results
Piecewise multilevel growth curve analyses indicated that maltreated/bullied and comparison children showed distinct cortisol responses to stress. Specifically, maltreated/bullied children had lower cortisol responses than comparison children who exhibited a significant increase. Lower cortisol responses were, in turn, associated with more social and behavioral problems among maltreated/bullied children.
Conclusions
These findings provide support for the influence of childhood harm on blunted HPAaxis reactivity and its potential impacton children’s functioning. Our findings emphasize the need to integrate stress biomarkers in guiding prevention efforts for young victims.
Keywords: Behavioral problems, bullying, cortisol, HPA axis, maltreatment, social problems
Evidence from animal and human studies suggests that the hypothalamic-pituitary-adrenal (HPA) axis contributes to physiological and behavioral adaptation to changing environments (1). Cortisol, the end product of the HPA axis, has widespread effects on the body, including enhancing glucogenesis and attention but also inhibiting the immune system, sexual motivation, and growth (2,3). Adaptive responses to stress are characterized by a relatively rapid cortisol increase followed by a progressive decline. Conversely, when secreted excessively, repeatedly, insufficiently, or in response to nonthreatening stimuli, cortisol may have detrimental effects over time (1,4). Research suggests that higher HPA axis reactivity, extensively documented in animal models of early-life stress (5,6), increases risk of depression in humans (7). Interest in the etiology and outcomes of lower HPA axis reactivity, also signaling a disruption of the HPA axis, is growing (8–10).
In addition to several studies reporting lower diurnal cortisol secretion in children exposed to early adversity (11–14), accumulating evidence suggests that these experiences may lead to lower cortisol responses to stress in adulthood (15–17). In comparison with control subjects matched for age and neighborhood, female adolescents with a history of childhood maltreatment showed lower cortisol responses to psychosocial stress (18). Consistent with these findings, women who experienced childhood abuse showed a reduced sensitivity of the adrenals to the adrenocorticotropic hormone in comparison with control subjects (19). Lower HPA axis reactivity has also been documented in the context of chronic concurrent stress. For example, female teachers experiencing chronic stress showed greater cortisol suppression to dexamethasone (20). Together, these findings suggest that early and concurrent chronic exposures to adverse life conditions may “get under the skin” and reduce HPA axis reactivity. The investigation of the association linking early-life stress and cortisol responses to psychosocial stress is, however, scarce and often relies on cross-sectional data of adults, retrospective measures of abuse, and small selected samples (e.g., psychiatric patients).
In contrast to the work conducted with adults, fewer studies have investigated the potential impact of blunted cortisol responses to psychological stress on social, emotional, and behavioral problems in childhood, when these problems are most likely to emerge. In childhood, depressive symptoms have been associated with both increased (21,22) and reduced (23,24) cortisol responses to psychological challenges. Recent findings also suggest a shift from lower cortisol responses among dysphoric children in the preschool years to higher responses in adolescence (25). Lower cortisol responses have also been associated with emotional and social problems in a community sample of 8- to 11-year-olds (26). Lower cortisol responses to psychosocial challenges are reported in school-aged children and adolescents with versus without behavioral problems (27–31), although mixed results are also found (32–34). Overall, these findings challenge the premise that only higher HPA axis reactivity signals difficulties in childhood and call for a broader investigation as to whether lower cortisol responses to stress may also have negative consequences.
One difficulty in interpreting the associations between cortisol responses to stress and social, emotional,and behavioral problems is that few studies have considered exposure to childhood abuse. Moreover, it is unclear whether similar associations should be expected between cortisol responses and psychological problems among maltreated versus nonmaltreated children. According to a diathesis-stress framework, the association between HPA axis reactivity and children’s difficulties could be context-dependant, whereby significant associations are more likely to emerge for children exposed to early adversity (35). Distinct patterns of associations between stress biomarkers and anxiety as a function of childhood adversity have been reported (36), suggesting that exposure to adverse environments should be considered as a potential moderator of the association between HPA axis reactivity and developmental outcomes.
This study had two objectives. First, because similar patterns of cortisol response to stress have been reported for childhood maltreatment (15–18) and bullying victimization (37), we examined whether 12-year-old maltreated/bullied children had lower cortisol responses to psychosocial stress than same-aged children. Childhood harm was prospectively and repeatedly measured throughout childhood. Second, we investigated whether lower cortisol responses were associated with social, emotional, and behavioral problems. We further tested whether the associations between cortisol responses and social, emotional, and behavioral problems were stronger among maltreated/bullied versus nonmaltreated/ bullied children.
Methods and Materials
Sample
Participants were recruited from the Environmental Risk (E-Risk) Longitudinal Twin Study, which tracks the development of a nationally representative birth cohort of 2232 British children (38) selected from a larger birth register of twins born in England and Wales in 1994 to 1995 (39). The original sample was constructed in 1999 to 2000, when 1116 families with same-sex 5-year-old twins (93% of those eligible) participated in home-visit assessments. Follow-up home visits were conducted when the children were aged 7 (98% participation), 10 (96% participation), and 12 years old (96% participation). Parents gave informed consent and children gave assent. Ethical approval was granted by the Joint South London and Maudsley and the Institute of Psychiatry National Health Service Ethics Committee (London, United Kingdom).
From the total E-Risk sample, we identified 190 12-year-old children (50.5% males) eligible to participate in a substudy of cortisol. Children were selected if they were identical twins and only one twin within the pair was bullied (see below). Most twins were Caucasian (91.6%) and one third of the families came from a low socioeconomic background (33.7%). Children had an IQ within the normal range when they were 5 years of age (from 64 to 142; mean [SD] = 101.38 [14.32]).
Procedure
The Psychosocial Stress Test (PST) took place at the research laboratory in the early afternoon (mean [SD] = 1:45 pm [22 minutes]). Each child was individually interviewed by a research assistant with a psychology degree. A video camera was installed in a room to record the cognitive and public speaking tasks adapted from the Trier Social Stress Test for Children (40). The cognitive task was first administered using the Children’s Paced Auditory Serial Addition Task (41). Children heard a random series of 61 numbers ranging from 1 to 9 and were instructed to add the numbers in pairs such that each number was added to the previous one. Participants were told to make as few mistakes as possible because they were in competition against their co-twin and the winner would get a prize. The interviewer did not offer support and avoided eye contact to enhance the stressful aspect of the laboratory challenge. For the public speaking task, children were asked to stand and recall their most unpleasant experience at school in front of an unknown and unexpressive judge and the interviewer. Children had 2 minutes to prepare in silence, standing up in front of the camera, and were then asked to speak for 5 minutes. The PST lasted approximately 13 minutes. At the end of the day, the interviewer told both the twins that they performed very well, rewarded their efforts, and allowed them to ask questions.
Main Outcomes
Cortisol was measured through the collection of five saliva samples. We asked children to use a straw to pass through 1 mL of saliva into the cryovials. The first two samples were collected 20 minutes and 2 minutes before the PST. A third sample was collected 2 minutes after the task. The fourth and fifth samples were collected 25 minutes and 35 minutes after the start of the task. Saliva samples were stored in a −20°C freezer and analyzed in a single batch using the DPC Inmmulite Immunoassay Analyzer (Siemens Healthcare Diagnostics, Munich, Germany; www.siemens.com) (42). The assay had an analytical sensitivity of .2 nmol/L and interassay/intra-assay precision of less than 10%.
History of Childhood Harm
Childhood harm consisted of either a history of childhood maltreatment or frequent bullying victimization. A total of 64 children from this substudy sample of 190 participants were harmed (33.7%); 23 were maltreated by an adult (12.1%), 27 were frequently bullied by their peers (14.2%), excluding those who were only occasionally bullied; and 14 were both maltreated and frequently bullied (7.4%).
Maltreatment was documented at each home visit, from ages 5 to 12, by interviewing mothers about past and ongoing physical harm that had happened to their children using the standardized clinical interview protocol from the Multi-Site Child Development Project, which has established validity and reliability in the E-Risk sample and others (43). Interviewers coded the likelihood that children had been maltreated based on mothers’ narratives. After each wave of the E-Risk Study, two clinical psychologists (T.E.M. and the project coordinator) separately reviewed each child’s cumulative dossier of information regarding harm gleaned from the successive home visits (i.e., from repeated caregiver interviews, debriefs with interviewers who coded any indication of child maltreatment, and from intervening clinicians when study made referral). Many, but not all, cases in the course of our research were under investigation by police or social services, already on the child-protection register, or in foster care at follow-up, having been removed from their parents because of abuse. The research team followed the guidelines for referral of families under the United Kingdom’s Children Act (44).
Bullying victimization was prospectively assessed for all E-Risk participants during the interviews conducted with mothers when children were 7, 10, and 12 years of age. We explained that, “Someone is being bullied when another child: says mean and hurtful things, makes fun or calls a person mean and hurtful names; completely ignores or excludes someone from their group of friends; hits, kicks, or shoves a person, or locks them in a room; tells lies or spreads rumors about them; and other hurtful things like these. We call it bullying when these things happen often and when it is difficult to make it stop. We do not call it bullying when it is done in a friendly or playful way.” We asked mothers whether each twin had been bullied by another child, responding “never,” “yes,” or “frequently,” An investigator reviewed all descriptions of the bullying events looking for evidence of repeated harmful actions, between children, where there was a power imbalance between the bully and the victim. A test-retest reliability of .87 was noted for 30 parents randomly selected from the total E-Risk sample and who were interviewed 3 to 6 weeks apart. Our findings indicate that mothers are valid and reliable informants of bullying victimization and that they tend to agree with their children’s reports (45).
Social, emotional, and behavioral problems at age 12 were assessed using the Child Behavior Checklist (CBCL) (46) for mothers and the Teacher’s Report Form (TRF) (47) for teachers. Mothers were given the instrument as a face-to-face interview and teachers responded by mail. Both informants rated each item as being 0 = not true, 1 = somewhat or sometimes true, or 2 = very true or often true. The reporting period was 6 months before the interview. The Social Problems scale is the sum of three items from each of the CBCL and the TRF, including items such as “he/she does not get along with other children.” The internal consistency reliability was .74 (range: 0–7; mean [SD] = 1.21 [1.63]) in this substudy sample. The Emotional Problems scale is the sum of 23 items from the CBCL and 27 items from the TRF on the Withdrawn and Anxious/Depressed scales, including items such as “cries a lot” and “worries.” The internal consistency reliability was .90 (range: 0–45; mean [SD] = 11.14 [9.07]) in this substudy sample. The Behavioral Problems scale is the sum of 35 items from the CBCL and 34 items from the TRF on the Delinquency and Aggression scales, including items such as “argues a lot” and “is cruel or nasty to other people.” The internal consistency reliability was .93 (range: 0–81; mean [SD] = 17.17 [14.81]) in this substudy sample. As expected, the three scales were significantly correlated (Social-Emotional: r = .59, p < .001; Social-Behavioral: r = .39, p < .001; Emotional-Behavioral: r = .34, p < .001) but tapped into different aspects of children’s functioning.
Individual Risk Factors and Covariates
A full description of the pre-existing, concomitant, and PST-related factors is available online (Supplement 1).
Statistical Analyses
We examined cortisol response using a piecewise latent growth curve model. This model offers the advantage of capturing nonlinear patterns of change and can be tailored to describe and predict intra-individual change before and following a theoretically meaningful point in time (48). Piecewise latent growth curve model estimates separately, but simultaneously, the mean level of cortisol at the end of the PST (intercept) and patterns of cortisol change during the baseline and response phases (Figure 1). The model contained both fixed and random estimates, corresponding to the parameters’ mean and variance across individuals. Models were fitted in Mplus Version 6.11 (Muthén & Muthén, Los Angeles, California) (49) using maximum likelihood estimation. We used the COMPLEX option in Mplus to compute adjusted standard error estimates correcting for the nonindependence of observations. All models were evaluated using recommended fit indices, including root mean square error of approximation, where values <.08 indicate acceptable fit and values <.05 indicate good fit; confirmatory fit index, where estimates >.90 indicate acceptable fit and values >.95 indicate good fit; and the standardized root mean square residual, where values <.08 are considered acceptable (50,51).
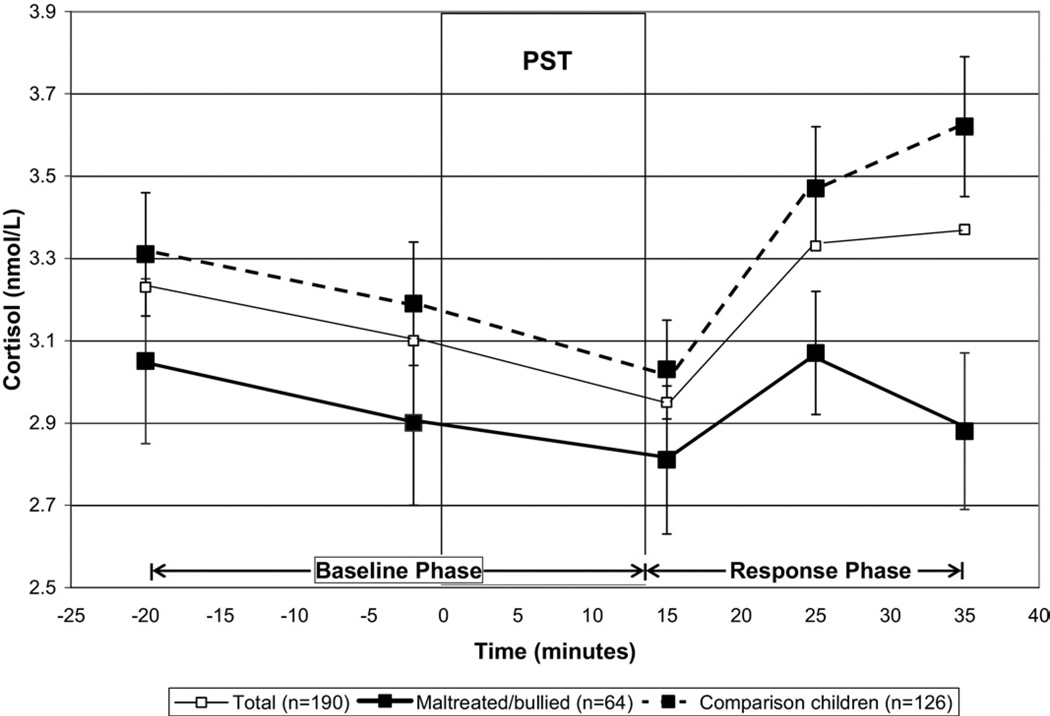
Figure 1.
Cortisol responses (± SEM) to the Psychosocial Stress Test (PST) in the total sample and according to maltreatment/bullying victimization.
We tested our objectives in three steps. First, we captured the pattern of cortisol response for the entire sample by estimating the intercept along with the baseline and response slopes. We then tested whether maltreated/bullied and comparison children showed distinct patterns of cortisol response to stress. Because maltreated/bullied children had increased emotional and behavioral problems at age 5 (Table S1 in Supplement 1), we subsequently controlled for these potential confounders. Second, we extracted the intercept, baseline, and response slope estimates derived in Mplus to test, using linear regression models, whether cortisol responses were associated with social, emotional, and behavioral problems. Third, we tested whether childhood harm moderated these associations by including an interaction term (cortisol response × childhood harm) in the regression models. We again controlled for age-5 social, emotional, and behavioral problems. Regression analyses were performed using STATA version 6 (Stata-Corp LP, College Station, Texas). To control for nonindependent observations, analyses were adjusted with tests based on the sandwich or Huber/White variance estimator (52).
Results
Do Children Show a Cortisol Response to the Psychosocial Stress Test?
Figure 1 illustrates the pattern of cortisol secretion before and following the PST for the total sample (solid thin line). Table 1 further specifies the fixed and random effect estimates of the mean cortisol levels at the end of the PST (intercept) and baseline and response slopes. During the baseline phase, children exhibited a significant cortisol decrease of .07 nmol/L per 10-minute interval (baseline slope) to reach a mean cortisol value of 2.97 nmol/L at the end of the PST. In response to the PST,children showed a significant mean cortisol increase of .21 nmol/L per 10-minute interval (response slope). The variance terms indicated that children varied significantly from one another on their cortisol levels at the end of the PST (intercept) and with respect to how they changed during the baseline and response phases. The covariance between the two slopes indicated that a more abrupt cortisol decrease in the baseline phase was associated with an elevated response to the PST. The influence of the baseline slope on cortisol response was thus controlled for in further analyses.
Table 1.
Fixed, Random, and Covariance Estimates of Mean Cortisol Levels at the End of the Psychosocial Stress Test and Cortisol Changes During the Baseline and Response Phases in the Total Sample of Children (n = 190)
| Statistics |
|||
|---|---|---|---|
| Parameters | B | SE | CR |
| Fixed (means) | |||
| Intercept (y0) | 2.97 | .13 | 23.43a |
| Baseline slope (yb) | −.07 | .03 | − 2.56b |
| Response slope (yr) | .21 | .05 | 4.49a |
| Random (variances) | |||
| Intercept (σ0) | 1.70 | .24 | 7.02a |
| Baseline slope (σb) | .05 | .01 | 3.32a |
| Response slope (σr) | .18 | .06 | 2.83c |
| Covariances | |||
| Intercept – Baseline slope (y0,yb) | −.04 | .05 | − .75 |
| Intercept – Response slope (y0,yr) | .11 | .09 | 1.25 |
| Baseline – Response slope (yb,yr) | .08 | .02 | 3.28a |
The CR refers to the ratio of the unstandardized beta estimate over the standard error (B/SE). Fit statistics: χ2 = 41.24, df = 10, CFI = .94, RMSEA = .13, SRMR = .07. The fixed estimate of the intercept represents the mean cortisol level at the end of the Psychosocial Stress Test, while the fixed estimates of the baseline and response slopes reflect the change of cortisol (nmol/L) per 10-minute interval.
B, unstandardized beta estimate; CFI, confirmatory fit index; CR, Critical Ratio; RMSEA, root mean square error of approximation; SE, standard error; SRMR, standardized root mean square residual.
p< .001.
p< .05.
p< .01.
Does Cortisol Response to Stress Differ Between Maltreated/ Bullied Versus Comparison Children?
Maltreated/bullied children did not differ from the comparison group with respect to their mean cortisol levels at the end of the PST (p = .48) or their rate of change in cortisol during the baseline phase (p = .85). However, we observed significant differences in cortisol responses between the two groups following the PST (p = .005) (Table 2, model 1). Figure 1 illustrates similar decreasing levels of cortisol before and during the PST in maltreated/bullied (bold solid line) and comparison children (bold dashed line). However, distinct patterns emerged thereafter; comparison children exhibited the expected increase in cortisol after the PST, whereas maltreated/ bullied children did not. This finding held when pre-existing emotional and behavioral problems were controlled for (Table 2, model 2). The model represented an adequate fit to the data (confirmatory fit index = .94, root mean square error of approximation = .11, standardized root mean square residual = .04).
Table 2.
Associations Between Childhood Harm (Maltreatment/Bullying) and Cortisol Levels (Intercept, Baseline Slope, Response Slope) During the Psychosocial Stress Test Controlling for Age-5 Emotional and Behavioral Problems (n = 190)
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Parameters | B | SE | CR | B | SE | CR |
| Childhood Harm → Intercept | − .19 | .26 | − .70 | −.09 | .26 | −.37 |
| Childhood Harm → Baseline Slope | .009 | .05 | .19 | −.01 | .05 | −.22 |
| Childhood Harm → Response Slope | − .23 | .08 | 2.79a | −.25 | .08 | −2.99a |
| Behavioral Pb → Intercept | −.01 | .01 | −1.05 | |||
| Behavioral Pb → Baseline Slope | .001 | .002 | .53 | |||
| Behavioral Pb → Response Slope | .001 | .004 | .39 | |||
| Emotional Pb → Intercept | −.01 | .01 | −.88 | |||
| Emotional Pb → Baseline Slope | .005 | .003 | 1.66 | |||
| Emotional Pb → Response Slope | .002 | .006 | .35 | |||
The CR refers to the ratio of the unstandardized beta estimate over the standard error (B/SE). Fit statistics of Model 1: χ2 = 45.00, df = 12, CFI = .94, RMSEA = .12, SRMR = .06; Model 2: χ2 = 55.27, df = 16, CFI = .94, RMSEA = .11, SRMR = .04.
B, unstandardized parameter estimate; CFI, confirmatory fit index; CR, critical ratio; Pb, problems; RMSEA, root mean square error of approximation; SE, standard error; SRMR, standardized root mean square residual.
p < .01.
Is Cortisol Response Associated with Social, Emotional, and Behavioral Problems and Is This Association Stronger Among Maltreated/Bullied Versus Comparison Children?
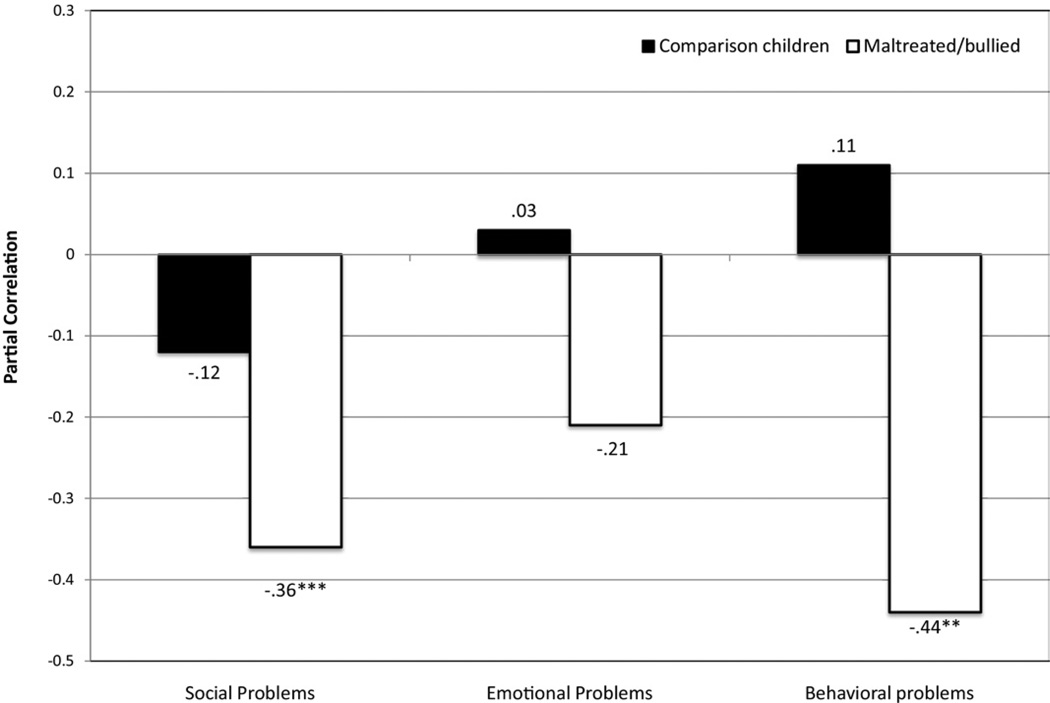
Table 3 (model 1) shows that childhood harm was significantly associated with children’s social, emotional, and behavioral problems. In contrast, lower cortisol responses were significantly associated with more social problems but not with emotional and behavioral problems. The moderated regression analyses in Table 3 (model 2) show that the associations between cortisol response and children’s social and behavioral problems were stronger among maltreated/bullied children than among comparison children. These interactions were statistically significant when predicting social and behavioral problems and remained significant when age-5 problems were included in the models (social problems: t = −2.56, p = .01; behavioral problems: t = −2.64, p = .01). Figure 2 shows that the associations between cortisol response to stress and children’s social, emotional, and behavioral problems were of moderate effect size and significant for social and behavioral problems among children who were maltreated/bullied. These associations were uniformly small and nonsignificant among comparison children.
Table 3.
Associations Between Cortisol Response and Children’s Social, Emotional, and Behavioral Problems at Age 12 According to Childhood Harm (Maltreatment/Bullying) (n = 190)
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| B | SE | t | B | SE | t | |
| Social Problems | ||||||
| Cortisol response | − 2.33 | .74 | − 3.13a | − 2.29 | .69 | −3.30b |
| Childhood harm | 1.02 | .26 | 3.93b | 1.29 | .30 | 4.30b |
| Cortisol response × childhood harm | −1.68 | .68 | −2.45c | |||
| Emotional Problems | ||||||
| Cortisol response | − 3.60 | 5.67 | −64 | −3.48 | 5.67 | −61 |
| Childhood harm | 3.92 | 1.44 | 2.72a | 4.66 | 1.54 | 3.02a |
| Cortisol response × childhood harm | −4.54 | 3.58 | −1.27 | |||
| Behavioral Problems | ||||||
| Cortisol response | −8.86 | 9.79 | −.90 | −8.47 | 9.57 | −.88 |
| Childhood harm | 6.09 | 2.73 | 2.23c | 8.39 | 2.96 | 2.84a |
| Cortisol response × childhood harm × Victimization | −14.14 | 4.79 | −2.95a | |||
Adjusted regression analyses controlling for sex, intercept and baseline slope. No significant gender moderation effects were detected. Therefore, all analyses were conducted on the combined sample of boys and girls.
B, unstandardized beta; SE, standard error.
p < .01.
p < .001.
p < .05.
Figure 2.
Associations between cortisol responses and children’s social, emotional, and behavioral problems as a function of maltreatment/bullying victimization (n = 190). Adjusted correlations controlling for sex, intercept, and baseline slope. ***p < .001; **p < .01.
Discussion
This study provides support for the hypothesis that early exposure to adverse life conditions could result in lower HPA axis reactivity to stress in childhood. Our laboratory stress paradigm elicited a significant cortisol response in comparison children but not in maltreated/bullied children for whom measures of harm were collected prospectively and repeatedly in childhood. We also showed that lower cortisol responses were associated with more social and behavioral difficulties but only among maltreated/bullied children. These findings suggest that blunted cortisol responses to stress may co-occur with social and behavioral problems, especially among maltreated/bullied children.
Our findings are consistent with a large body of evidence highlighting lower cortisol responses to stress in individuals who faced early-life stress (8,9,16–18,53). We extended these findings in three ways. First, we showed that low cortisol responses in participants exposed to childhood harm can already be detected at 12 years and are thus not restricted to late adolescence and adulthood. This is consistent with lower cortisol response to stress noted in children exposed to interparental conflict (31), suggesting that the lowering effect of adverse experiences on HPA axis reactivity may not be limited to direct victimization. Second, although maltreated/bullied children had more emotional and behavioral difficulties than comparison children at age 5,these pre-existing differences did not account for the lower cortisol responses shown in maltreated/bullied children. Controlling for individual characteristics was important, as these traits may elicit negative parental practices and bullying victimization (54,55). Third, we showed that childhood harm was associated with lower cortisol responses to stress, which signaled increased social and behavioral problems among maltreated/ bullied children.
This investigation showed that lower cortisol responses to stress were associated with social and behavioral problems only among maltreated/bullied children. Blunted HPA axis reactivity may therefore point to increased developmental risks for individuals confronting childhood harm. This pattern of results is consistent with other reports suggesting that the associations between disrupted HPA axis reactivity and social and emotional difficulties vary as a function of early-life stress (35). Previous research showed that depressed adolescents with a history of maltreatment had lower cortisol responses to stress than minimally depressed or healthy participants. Conversely, depression severity was not associated with cortisol responses among nonmaltreated participants (56). Taken together, these studies suggest that lower HPA axis reactivity may signal psychological problems among individuals exposed to childhood maltreatment.
There is also evidence that early adversity moderates the relationship between cortisol responses to stress and social outcomes (57). Preschoolers who showed high cortisol responses were less prosocial than control subjects when living in high familial adversity but tended to be more prosocial than control subjects when exposed to low familial adversity. Notably, higher prosocial behaviors were observed for lower responders when exposed to high familial adversity. One hypothesis is that participants’ age may explain these findings. A shift from increased (58) to reduced (29,30,59,60) cortisol responses to psychosocial stress is typically noted between preschoolers and adolescents with behavioral problems. A similar shift from increased to reduced cortisol secretion has also been documented among children and adults confronted with childhood abuse (61,62). This pattern of cortisol change over time is consistent with the attenuation hypothesis, which posits that persisting activations eventually downregulate HPA axis reactivity to limit repeated physiological, emotional, and behavioral responses to stress (63,64). Whether preschoolers experiencing chronic adversity are more likely to show lower HPA axis reactivity and greater behavioral problems later in life should be examined prospectively.
Moderating effects of early adversity have also been reported in the context of diurnal cortisol secretion, where higher morning cortisol was associated with more problematic functioning in non-maltreated but not in maltreated children (65). Stronger associations were also found between diurnal cortisol and aggression among nonmaltreated versus maltreated children (66). These findings suggest the need to better characterize the potential moderating influence of adverse childhood experiences in the investigation of the physiological correlates of children’s functioning.
The present study suggests that adverse experiences resulting in lower HPA axis reactivity may place individuals at greater risk for social and behavioral problems. Although the mechanisms involved remain uncertain, several hypotheses can be put forward. Environmentally induced alterations in brain structures regulating HPA axis reactivity (e.g., amygdala, hypothalamus) could affect both cortisol responses to stress and behaviors (1,3). For example, altered amygdala activity likely affects emotional responses to threats, acquisition of conditioned fears, and apprehension of punishment (67), landmarks of persistent externalizing problems (64,68–70). Lower cortisol responses to stress have also been proposed to interfere with children’s functioning through reduced sensitivity to punishment (71), decreased inhibition of socially inappropriate behaviors (72), and negative mood fluctuations under stress (73). Perhaps central to these difficulties is the reduced ability to maintain positive engagement in increasingly complex social environments (9). Children need to integrate several high-order cognitive and affective processes regulated by brain structures receptive to cortisol to interact positively with their peers. Lower cortisol secretion under stress and interconnected suboptimal functioning of brain structures regulating the HPA axis could jeopardize maltreated/bullied children’s capacity to establish healthy social relationships. Studies integrating biological markers present at multiple levels of analysis (e.g., cortisol, functional magnetic resonance imaging) are needed to better understand the underpinnings of social and behavioral problems among maltreated/bullied children.
The present findings should be considered in light of some limitations. First, it is possible that lower cortisol response reflects the influence of genetic factors rather than environmental influences. Exposure to maltreatment and bullying has been shown to have an environmentally-mediated effect on children’s mental health (74,75), which suggests that childhood harm could have a similar effect on HPA axis reactivity. Second, we measured cortisol on only one occasion and could not test whether differences in cortisol responses to stress were present before childhood harm. However, because we assessed the confounding effect of a wide range of early characteristics, we speculate that this alternative is unlikely. Third, our cortisol assessment would have benefited from a longer relaxation period before the stress paradigm, as stable pretest levels were not reached. An additional posttest saliva sample would have also allowed a better characterization of the distinct response patterns between the two groups.
We found that maltreated/bullied children had a blunted cortisol response to stress and that this lack of response was associated with increased social and behavioral problems. These findings have implications for research and clinical practice. First, future research investigating HPA axis reactivity and behavior can benefit from adopting a diathesis-stress framework. Early-life exposure to adversity involving childhood harm could serve as a measure of diathesis in future studies and may reveal larger effects of lower HPA axis reactivity on behavior. Second, prevention and intervention efforts should integrate cortisol as a part of their overall assessment and test whether changes in cortisol are associated with increased psychological resilience or improvement in social and behavioral problems among maltreated/bullied children. Preliminary findings suggest that attachment-based and family-based preventive intervention successfully normalizes HPA axis reactivity in high-risk children (76,77), which, in turn, mediates a greater decline in aggression (78). Better understanding of the biological, emotional, and cognitive mechanisms underlying the developmental risk associated with blunted HPA axis reactivity is needed to inform prevention efforts among at-risk children.
Supplementary Material
Acknowledgments
The Environmental Risk Longitudinal Twin Study is funded by the Medical Research Council (G9806489 and G1002190). Additional support was provided by the Jacobs Foundation, the British Academy, the Nuffield Foundation, the Economic and Social Research Council (RES-177-25-0013), and National Institute of Child Health and Human Development (HD061298). Isabelle Ouellet-Morin is supported by the Canadian Institutes of Health Research. Candice L. Odgers is a William T. Grant Scholar. Andrea Danese is supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award; Sania Shakoor is supported by the Medical Research Council; and Avshalom Caspi is a Royal Society Wolfson Research Merit Award holder.
We are grateful to the families and the twins’ teachers for their participation. Our thanks to Michael Rutter and Robert Plomin, to Thomas Achenbach for kind permission to adapt the Child Behavior Checklist, to Irene Papadopoulos for technical assistance, and to members of the Environmental Risk Longitudinal Twin Study team for their dedication, hard work, and insights.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 3.Schulkin J. Allostasis: A neural behavioral perspective. Horm Be-hav. 2003;43:21–27. doi: 10.1016/s0018-506x(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Protective and damaging effects of the mediators of stress and adaptation: Allostasis and allostatic load. In: Schulkin J, editor. Allostasis, Homeostasis, and the Costs of Physiological Adaptation. Cambridge, UK: Cambridge University Press; 2004. pp. 65–98. [Google Scholar]
- 5.Suomi SJ. Early determinants of behaviour: Evidence from primate studies. Br Med Bull. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- 6.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psy-choneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 9.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 10.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- 12.Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. Effects of therapeutic interventions for foster children on behavioral problems, caregiver attachment, and stress regulatory neural systems. Ann N Y Acad Sci. 2006;1094:215–225. doi: 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- 13.Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, et al. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreat. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- 14.Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Dev Psychobiol. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpen-ter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: A study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, et al. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the Youth Mood Project. Biol Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 20.Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dockray S, Susman EJ, Dorn LD. Depression, cortisol reactivity, and obesity in childhood and adolescence. J Adolesc Health. 2009;45:344–350. doi: 10.1016/j.jadohealth.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- 24.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: Evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 2004;161:1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- 25.Hankin BL, Badanes LS, Abela JR, Watamura SE. Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biol Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyrka AR, Kelly MM, Graber JA, DeRose L, Lee JK, Warren MP, Brooks-Gunn J. Behavioral adjustment in a community sample of boys: Links with basal and stress-induced salivary cortisol concentrations. Psychoneuroendocrinology. 2010;35:1167–1177. doi: 10.1016/j.psyneuen.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Popma A, Jansen LM, Vermeiren R, Steiner H, Raine A, Van Goozen SH, et al. Hypothalamus pituitary adrenal axis and autonomic activity during stress in delinquent male adolescents and controls. Psychoneuroendocrinology. 2006;31:948–957. doi: 10.1016/j.psyneuen.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Snoek H, Van Goozen SH, Matthys W, Buitelaar JK, van Engeland H. Stress responsivity in children with externalizing behavior disorders. Dev Psychopathol. 2004;16:389–406. doi: 10.1017/s0954579404044578. [DOI] [PubMed] [Google Scholar]
- 30.van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wie-gant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biol Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- 31.Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The role of child adrenocortical functioning in pathways between interparental conflict and child maladjustment. Dev Psychol. 2007;43:918–930. doi: 10.1037/0012-1649.43.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- 33.McBurnett K, Raine A, Stouthamer-Loeber M, Loeber R, Kumar AM, Kumar M, Lahey BB. Mood and hormone responses to psychological challenge in adolescent males with conduct problems. Biol Psychiatry. 2005;57:1109–1116. doi: 10.1016/j.biopsych.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 34.Alink LR, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev Psychobiol. 2008;50:427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- 35.Boyce TW, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 36.van der Vegt EJ, van der Ende J, Huizink AC, Verhulst FC, Tiemeier H. Childhood adversity modifies the relationship between anxiety disorders and cortisol secretion. Biol Psychiatry. 2010;68:1048–1054. doi: 10.1016/j.biopsych.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Ouellet-Morin I, Danese A, Bowes L, Shakoor S, Ambler A, Pariante C, et al. AdiscordantMZtwin design shows blunted cortisol reactivity among bullied children. J Am Acad Child Adolesc Psychiatry. 2011;50:574–582. doi: 10.1016/j.jaac.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.E-Risk Study Team. Moffitt TE. Teen-aged mothers in contemporary Britain. J Child Psychol Psychiatry. 2002;43:727–742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- 39.Trouton A, Spinath FM, Plomin R. Twins Early Development Study (TEDS): A multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Res. 2002;5:444–448. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- 40.Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Dyche G, Johnson D. Development and evaluation of CHIPASAT, an attentional test for children: II. Test-retest reliability and practice effect for a normal sample. Percept Mot Skills. 1991;72:563–572. doi: 10.2466/pms.1991.72.2.563. [DOI] [PubMed] [Google Scholar]
- 42.Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D’Albenzio A, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: The role of stress and of antipsy-chotic treatment. Schizophr Res. 2010;116:234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- 44.Department of Health. The Children Act. London: HMSO; 1989. [Google Scholar]
- 45.Shakoor S, Jaffee SR, Andreou P, Bowes L, Ambler AP, Caspi A, et al. Mothers and children as informants of bullying victimization: Results from an epidemiological cohort of children. J Abnorm Child Psychol. 2011;39:379–387. doi: 10.1007/s10802-010-9463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Achenbach TM. Manual for the Child Behavior Checklist 4–18 and Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 47.Achenbach TM. Manual for the Teacher’s Report Form and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 48.Flora DB. Specifying piecewise latent trajectory models for longitudinal data. Struct Equ Modeling. 2008;15:513–533. [Google Scholar]
- 49.Muthén M. Mplus. Los Angeles: Muthén & Muthén; 1998–2008. [Google Scholar]
- 50.McDonald RP, Ho MH. Principles and practice in reporting structural equation analyses. Psychol Methods. 2002;7:64–82. doi: 10.1037/1082-989x.7.1.64. [DOI] [PubMed] [Google Scholar]
- 51.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 52.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 53.Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boivin M, Perusse D, Dionne G, Saysset V, Zoccolillo M, Tarabulsy GM, et al. The genetic-environmental etiology of parents’ perceptions and self-assessed behaviours toward their 5-month-old infants in a large twin and singleton sample. J Child Psychol Psychiatry. 2005;46:612–630. doi: 10.1111/j.1469-7610.2004.00375.x. [DOI] [PubMed] [Google Scholar]
- 55.Bowes L, Arseneault L, Maughan B, Taylor A, Caspi A, Moffitt TE. School, neighborhood, and family factors are associated with children’s bullying involvement: A nationally representative longitudinal study. J Am Acad Child Adolesc Psychiatry. 2009;48:545–553. doi: 10.1097/CHI.0b013e31819cb017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunnar MR, Sebanc AM, Tout K, Donzella B, van Dulmen MM. Peer rejection, temperament, and cortisol activity in preschoolers. Dev Psy-chobiol. 2003;43:346–358. doi: 10.1002/dev.10144. [DOI] [PubMed] [Google Scholar]
- 59.van Goozen SH, Matthys W, Cohen-Kettenis PT, Buitelaar JK, van Enge-land H. Hypothalamic-pituitary-adrenal axis and autonomic nervous system activity in disruptive children and matched controls. J Am Acad Child Adolesc Psychiatry. 2000;39:1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- 60.Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 62.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 64.Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Dev Psychopathol. 2007;19:787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray-Close D, Han G, Cicchetti D, Crick NR, Rogosch FA. Neu-roendocrine regulation and physical and relational aggression: The moderating roles of child maltreatment and gender. Dev Psychol. 2008;44:1160–1176. doi: 10.1037/a0012564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 68.Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Raine A, Venables PH, Mednick SA. Low resting heart rateatage 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. J Am Acad Child Adolesc Psychiatry. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- 70.Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: Follow-up at age 26 years. Dev Psychopathol. 2002;14:179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- 71.Lupien SJ, Ouellet-Morin I, Hupbacha A, Tu MT, Buss C, Walker D, et al. Beyond the stress concept: Allostatic load-a developmental biological and cognitive perspective. In: Cicchetti D, Donald JC, editors. Developmental Psychopathology. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2006. pp. 578–628. [Google Scholar]
- 72.Oosterlaan J, Geurts HM, Knol DL, Sergeant JA. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry Res. 2005;134:1–10. doi: 10.1016/j.psychres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Het S, Wolf OT. Mood changes in response to psychosocial stress in healthy young women: Effects of pretreatment with cortisol. Behav Neurosci. 2007;121:11–20. doi: 10.1037/0735-7044.121.1.11. [DOI] [PubMed] [Google Scholar]
- 74.Jaffee SR, Caspi A, Moffitt TE, Taylor A. Physical maltreatment victim to antisocial child: Evidence of an environmentally mediated process. J Abnorm Psychol. 2004;113:44–55. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- 75.Arseneault L, Milne BJ, Taylor A, Adams F, Delgado K, Caspi A, Moffitt TE. Being bullied as an environmentally mediated contributing factor to children’s internalizing problems: A study of twins discordant for victimization. Arch Pediatr Adolesc Med. 2008;162:145–150. doi: 10.1001/archpediatrics.2007.53. [DOI] [PubMed] [Google Scholar]
- 76.Dozier M, Peloso E, Lewis E, Laurenceau JP, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Dev Psychopathol. 2008;20:845–859. doi: 10.1017/S0954579408000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brotman LM, Gouley KK, Huang KY, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Arch Gen Psychiatry. 2007;64:1172–1179. doi: 10.1001/archpsyc.64.10.1172. [DOI] [PubMed] [Google Scholar]
- 78.O’Neal CR, Brotman LM, Huang KY, Gouley KK, Kamboukos D, Calzada EJ, Pine DS. Understanding relations among early family environment, cortisol response, and child aggression via a prevention experiment. Child Dev. 2010;81:290–305. doi: 10.1111/j.1467-8624.2009.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.