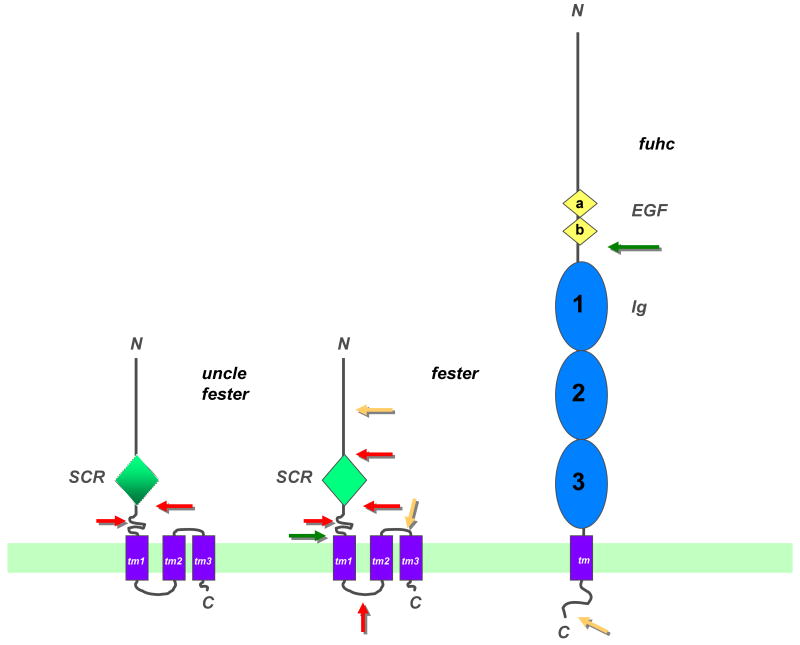
Figure 2.
Topological models of uncle fester, fester and the fuhc proteins. Both fester and uncle fester encode an extracellular SCR domain (green diamond), although there is no amino acid homology between the two. This is followed by three predicted transmembrane domains and an intracellular COOH tail. Portions of the molecule deleted by alternative splicing are shown (arrows). Red arrows show regions which are spliced frequently, yellow arrows are regions which are spliced rarely. The green arrow on fester depicts a splice variant which removes all the transmembrane domains, creating a putatively secreted form of the protein (see text for details). The fuhc protein is shown on the right, with two tandem EGF domains (yellow diamonds), followed by three tandem immunoglobulin (C2 type) domains (blue ovals), followed by a TM region and short intracellular tail. Two splice variants of the fuhc have been detected; the green arrow shows a splice which creates a putative secreted form of the protein, containing ca. 50% of the ectodomain, while the yellow arrow indicates a region where a small exon is inserted in ca. 5% of the transcripts, however it encodes no obvious motifs. These structures are based on predictions from multiple algorithms, but have not been directly demonstrated [13]. For example, one program predicts an Ig domain at the N-terminus of fester, and two Ig domains at the N-terminus of the fuhc, preceding the EGF domains (F. Bazan, personal communication). In addition, Ig domain 1 is predicted by some, but not all programs.