Abstract
OBJECTIVE
Short leg length, a marker of early childhood deprivation, has been used in studies of the association of early life conditions with adult chronic disease risk. The objective of this study was to determine the cross-sectional associations of leg length with measures of insulin sensitivity and β-cell function.
RESEARCH DESIGN AND METHODS
Subjects (n = 462) at risk for type 2 diabetes were recruited into the PROspective Metabolism and ISlet cell Evaluation (PROMISE) longitudinal cohort. Leg length was calculated from sitting and standing height at the 3-year clinical examination. Glucose tolerance status was determined using an oral glucose tolerance test. Insulin sensitivity was assessed using homeostasis model assessment of insulin resistance (HOMA-IR) and the Matsuda insulin sensitivity index (ISI), while the insulinogenic index over HOMA-IR (IGI/IR) and the insulin secretion sensitivity index 2 (ISSI-2) determined β-cell function. Multiple linear regression analysis was conducted, adjusting for covariates including age, sex, ethnicity, family history of diabetes, waist, and weight.
RESULTS
Leg length and leg-to-height ratio were significantly associated with HOMA-IR (β = −0.037, β = −10.49, respectively; P < 0.0001), ISI (β = 0.035, β = 8.83, respectively; P < 0.0001), IGI/IR (β = 0.021, P < 0.05; β = 7.60, P < 0.01, respectively), and ISSI-2 (β = 0.01, P < 0.03; β = 3.34, P < 0.01, respectively) after adjustment for covariates. The association of shorter leg length with lower insulin sensitivity was most evident for those with high waist circumferences.
CONCLUSIONS
Shorter legs were independently associated with lower insulin sensitivity and β-cell function, suggesting that early childhood deprivation may increase the risk of developing diabetes.
Over 285 million individuals worldwide are afflicted with type 2 diabetes (1). The increasing prevalence of this condition and its associated comorbidities represent a significant public health concern. Type 2 diabetes is a complex, multifactorial disease characterized by a decrease in both β-cell function and insulin sensitivity, the underlying causes of which have not been fully elucidated. An emerging hypothesis in the study of the natural history of type 2 diabetes focuses on the role of early life deprivation (2); this hypothesis posits that environmental conditions such as poor nutrition, stress, and infection during early life compromise later adult health and increase the risk for chronic diseases.
The period between 0 and 4 years of age is considered a nutritionally dependent phase of growth (3). During this period, growth occurs predominately in the head and the legs (4,5). Nutritional deprivation or stressful circumstances during this time period can interrupt growth, permanently affecting the development of the legs and other organs. Low socioeconomic status (SES) during childhood (6,7), low parental education (8,9), displacement during infancy because of war (10), not being breast-fed or having a lower energy intake during childhood (7) have been shown to be associated with shorter adult leg length, independent of birth weight (11). Thus, leg length may be a useful marker of early childhood conditions when studying the impact of early life deprivation on adult disease risk.
A number of previous articles have reported inverse associations of leg length with type 2 diabetes prevalence and incidence (12–17), though there have been some inconsistencies in the literature (18,19). In addition, a limited number of investigations have evaluated the association of leg length with metabolic disorders underlying type 2 diabetes; while some studies found inverse relationships of leg length with insulin resistance (12,13,20,21), the findings have not been entirely consistent (19). Of note, these studies have used simpler, fasting-based measures of insulin resistance (i.e., homeostasis model assessment of insulin resistance [HOMA-IR]), with none using more detailed measures of insulin sensitivity or assessing β-cell function. The lack of information regarding associations with β-cell function, defined as the compensatory relationship between insulin secretion and sensitivity, is a particularly important limitation given the central role of this disorder in the pathogenesis of type 2 diabetes. In addition, there may be potential interactions between stature components and other risk factors, such as waist circumference (which reflects current adult metabolic status), that may highlight the match-mismatch between early and late life (2,22), although to our knowledge this has not been investigated in the literature. Therefore, the objectives of this study were to determine the associations of leg length with insulin sensitivity and β-cell function in adults at risk for type 2 diabetes and to test for potential interactions with other risk factors for type 2 diabetes (including waist circumference). We hypothesized that shorter leg length would be associated with poorer insulin sensitivity and β-cell function and that shorter legs and larger waist circumferences would display the poorest insulin sensitivity and β-cell function in this at-risk population.
RESEARCH DESIGN AND METHODS
Data used for this article were from the 3-year follow-up examination (2007–2009) of the PROspective Metabolism and ISlet cell Evaluation (PROMISE) study (23,24), which is a longitudinal observational cohort study involving subjects with one or more risk factors for type 2 diabetes, including obesity, hypertension, family history of type 2 diabetes, or a history of gestational diabetes mellitus or birth of a macrosomic infant. At baseline (2004–2006), participants ≥30 years of age were recruited from Toronto and London, Ontario, Canada, through poster or newspaper advertisements (n = 654). Participants in the cohort undergo extensive metabolic characterization, anthropometric measurements, and lifestyle questionnaires every 3 years. Between the baseline visit and the first follow-up (3 year) visit, 25 subjects developed diabetes, 80 withdrew, 47 were contacted but did not attend the visit, 21 were lost to follow-up, and 19 did not have complete data. Only participants without diabetes at the 3-year examination, based on the 1999 World Health Organization criteria (25), who had completed an oral glucose tolerance test (OGTT) and had complete data on primary exposures and outcomes, were included in the current cross-sectional analysis (n = 462). The study received ethics approval from the participating institutions.
Anthropometric measures were determined using standard procedures. Height was measured with the subject standing against a wall-mounted stadiometer without shoes. Sitting height was included at the 3-year follow-up visit and was measured with the subject sitting on a nonpadded, fixed-height stool against the wall-mounted stadiometer with back straight and head in the Frankfurt plane. Sitting height includes the head, neck, and trunk. Waist circumference was measured at the natural waist, identified as the narrowest part of the torso between the umbilicus and the xiphoid process. All measurements were taken twice, and the average was used in the analysis. Subischial leg length was calculated by subtracting sitting height (minus the stool height) from standing height. This method of estimating leg length from sitting height has been used previously in epidemiological studies (13,20). Leg-to-height ratio (LHR) was calculated by dividing leg length by height, a measure that has also been used in previous studies (15,17).
Structured questionnaires assessed sociodemographics (life occupation, education, and parental education) and self-reported ethnicity and sex, as well as personal and family health history (family history of type 2 diabetes, self-reported weight at 18 years, self-reported birth weight, and presence of other chronic diseases, which included having a history of myocardial infarction, stroke, polycystic ovarian syndrome, hypertension, known high cholesterol, peripheral arterial disease, kidney disease, thyroid disease, or cancer).
Metabolic characterization involved an 8- to 12-h overnight fasted blood sample, followed by a 75-g OGTT, with additional blood samples being drawn at 30 and 120 min. Blood samples were processed and frozen at −70°C for the determination of insulin and other biomarkers. Specific insulin was measured using the Elecsys 1010 (Roche Diagnostics, Basel, Switzerland) immunoassay analyzer and electrochemiluminescence immunoassay. Standard laboratory procedures were used to determine glucose. Insulin resistance was assessed using HOMA-IR (26) calculated by dividing the product of fasting glucose and insulin by 22.5. Insulin sensitivity was assessed using the Matsuda insulin sensitivity index (ISI) for OGTTs (27), calculated by dividing 10,000 by the square root of the products of fasting glucose and insulin and the average OGTT levels of glucose and insulin. HOMA-IR largely reflects hepatic insulin resistance while ISI measures whole-body insulin sensitivity (28). Measures of β-cell function that were used take into consideration the compensatory relationship between insulin secretion and insulin sensitivity, which were the insulinogenic index (29) over HOMA-IR (IGI/IR) and the insulin secretion sensitivity index-2 (ISSI-2) (30). The insulinogenic index was calculated by dividing the difference of 30-min insulin and fasting insulin by the difference of 30-min glucose and fasting glucose. ISSI-2 was calculated by dividing the insulin area under the curve (AUC) by the glucose AUC and multiplying by ISI. Glucose and insulin AUCs were determined from OGTT values using the trapezoidal rule. IGI/IR is a measure of first-phase insulin secretion, while the more recently developed ISSI-2 is analogous to the disposition index but is estimated from OGTT data. These insulin resistance/sensitivity and β-cell function indices have been validated against gold standard measures (26,27,30).
Statistical analysis was conducted using SAS 9.3 for Linux (SAS Institute, Cary, NC), and the ggplot2 package (31) in R (32) was used for the graphics. Analyses were conducted using the 3-year visit data of the PROMISE cohort (n = 462, 2007–2009), as sitting height was only measured at the 3-year visit. The primary outcome variables were HOMA-IR, ISI, IGI/IR, and ISSI-2. The main exposure variables included height, sitting height, leg length, and LHR, as each are thought to represent different phases of growth (6). As proposed by others (33), SES was calculated by summing together the occupation and education scales and categorizing four groups based on the ranges of the sums (“lowest” SES is the sum of the occupation and education scales ranging from 0 to 2, “low” is from 3 to 4, “high” is from 5 to 6, and “highest” is from 7 to 9). While income and birth weight questions were included on the questionnaire, there were a large number of missing, decline to respond, and “unknown” answers (n = 96 and n = 107 at the 3-year visit, respectively); thus, income and birth weight were not included in the analysis to maintain sufficient power.
Anthropometrics, age, insulin sensitivity, and β-cell function measures were analyzed as continuous variables while sex, ethnicity, SES, family history of type 2 diabetes, presence of other chronic diseases, and parental education were analyzed as discrete variables. Stature components were tested for differences across categories of discrete variables using ANCOVA, adjusted for ethnicity, sex, and age, followed by post hoc Tukey pairwise testing in the case of significant between-group findings. Continuous variables were analyzed using Spearman partial correlation, adjusted for ethnicity, sex, and age.
Multiple linear regression analysis was used to assess the association of the components of stature with the primary outcome variables, after adjustment for covariates. Regression residuals were nonnormally distributed, and log transformation of insulin sensitivity and β-cell function measures corrected normality. Model covariates were selected based on a significant association with the stature components or because of previous documentation in the literature (12,13,20,21). Covariates in sequentially nested models included ethnicity, sex, and age (model 1) to control for inherent characteristics; model 1 variables plus SES and parental education (model 2) to control for both current and early life socioeconomic conditions; model 2 plus presence of chronic disease and family history of type 2 diabetes (model 3) to control for the influence that other diseases, personal and familial, may have on health; and model 3 plus weight and waist circumference (model 4) to control for current health status. In addition, three sensitivity analyses were conducted by 1) replacing the stature component exposure variables with sex- and ethnicity-standardized z scores of stature components, 2) adjusting for height in the leg length models to compare with the LHR models, and 3) including weight at 18 years in model 4 as a measure of end of childhood health status.
For determination of whether the association of leg length or LHR with measures of insulin sensitivity and β-cell function differed according to preidentified demographic and anthropometric factors, interaction terms for sex, ethnicity, parental education, waist circumference, and weight were tested with stature components as continuous variables. Interaction terms were tested using ANCOVA in a minimally adjusted model (model 1). For these analyses, waist circumference and weight were categorized into quartiles (n = 114–117 for each quartile).
RESULTS
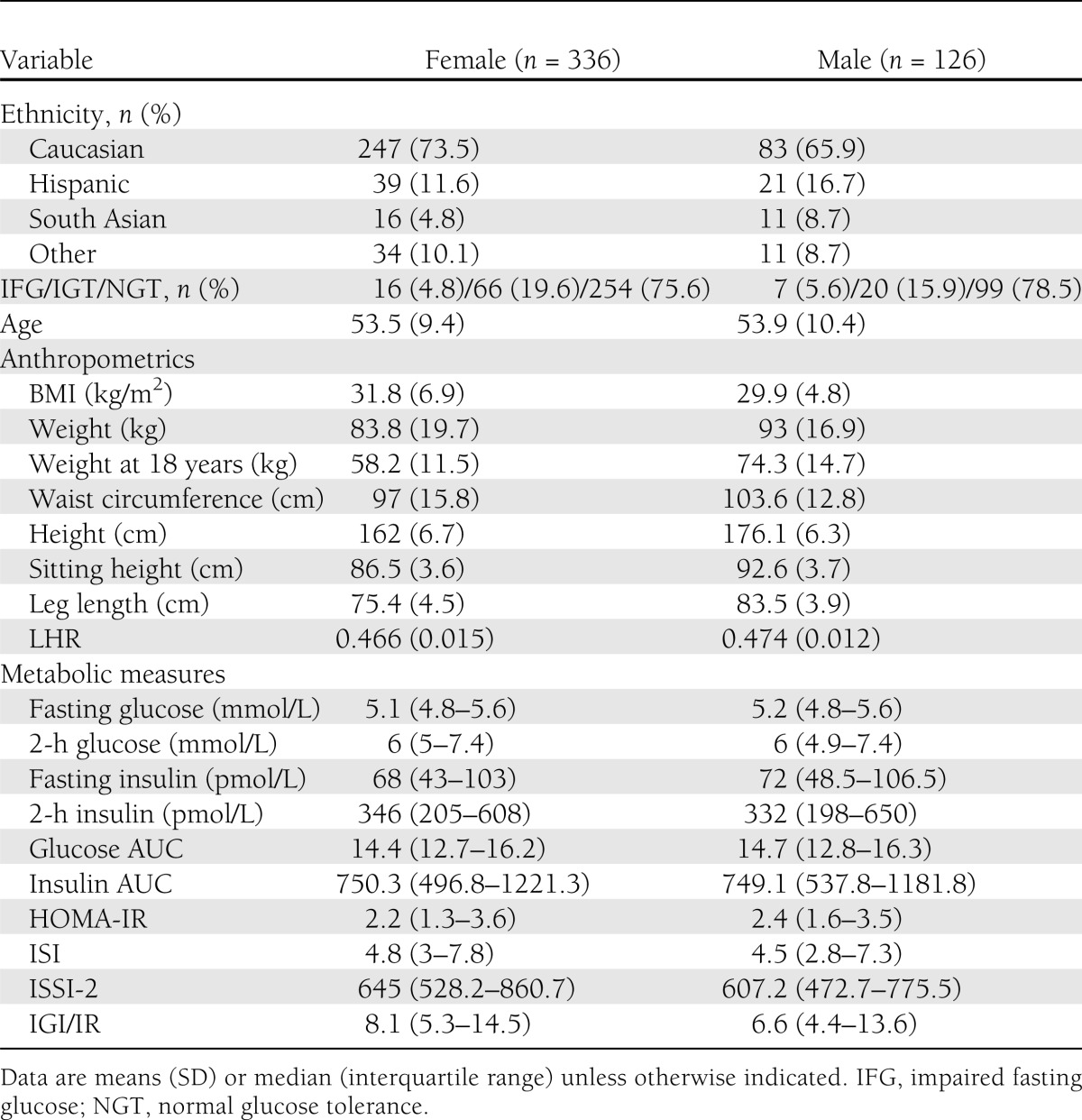
Metabolic and anthropometric characteristics of participants are presented in Table 1 by sex. Mean age of both female and male participants was 54 years. The study population comprised 336 (73%) females and 330 (71%) Caucasians, with smaller proportions of Hispanics and South Asians (13 and 6%, respectively). Seventy-four percent (n = 353) of subjects had normal glucose tolerance, 18.6% (n = 86) had impaired glucose tolerance (IGT), and 5% (n = 23) had impaired fasting glucose.
Table 1.
Metabolic, demographic, and anthropometric characteristics from 3-year visit (2007–2009) of nondiabetic subjects from the PROMISE cohort
Weight, weight at 18 years of age, and waist circumference were significantly correlated with leg length (r = 0.31, r = 0.36, and r = 0.18, respectively; all P < 0.0003), height (r = 0.40, r = 0.38, and r = 0.22, respectively; all P < 0.0001), sitting height (r = 0.36, r = 0.26, and r = 0.18, respectively; all P < 0.0002), and LHR (r = 0.09 and r = 0.21 for weight and weight at 18 years of age, P < 0.05; not significant for waist circumference, r = 0.08, P = 0.10) based on Spearman partial correlation results. Age was significantly correlated with sitting height (r = −0.32), height (r = −0.16), and LHR (r = 0.24, all P < 0.0005), although there were no significant correlations with leg length.
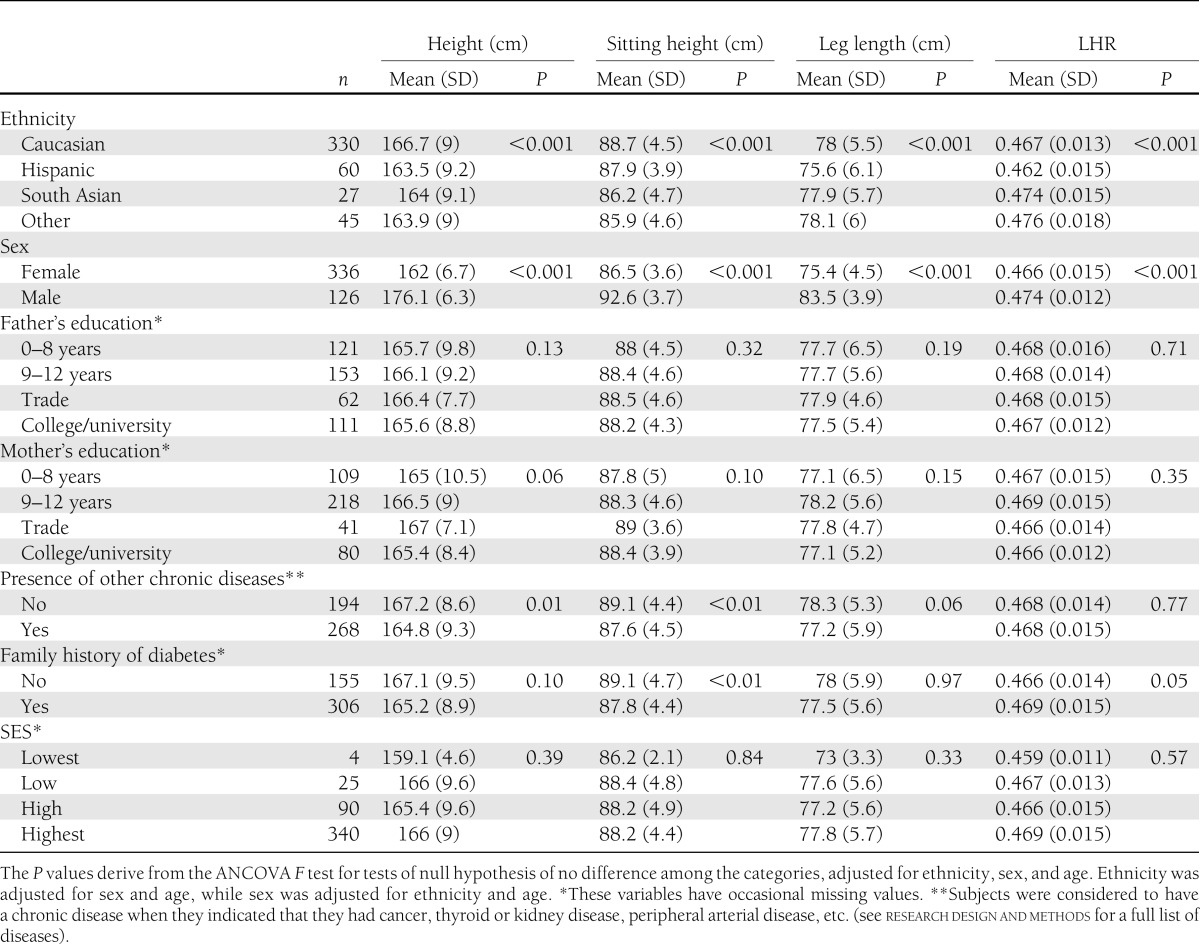
The means of each stature component according to categories of anthropometric and demographic variables are presented in Table 2. Males had significantly longer legs and sitting heights, were taller, and had higher LHR (all P < 0.0001) after adjustment for ethnicity and age. Caucasians were taller (P < 0.02) and had longer sitting heights (P < 0.0001) than all other ethnicities and had lower LHR than “other ethnicities” (P = 0.0001). Hispanics had shorter legs than “other ethnicities” (P = 0.0005) and had lower LHR than South Asian and “other ethnicities” (P < 0.002). No significant differences were seen according to father's education. Subjects whose mother's education had been in the trades were taller than subjects with mothers who had between 0 and 8 years of education (P = 0.05). Those with chronic diseases (i.e., cancer, peripheral arterial disease, thyroid disease, etc.) were significantly shorter (P = 0.01) and had shorter legs (P < 0.01) and sitting heights (P = 0.057), while those with a family history of type 2 diabetes had shorter sitting heights and higher LHR (all P < 0.05).
Table 2.
Means of stature components according to categories of demographic variables from nondiabetic subjects in the 3-year visit (2007–2009) of the PROMISE cohort
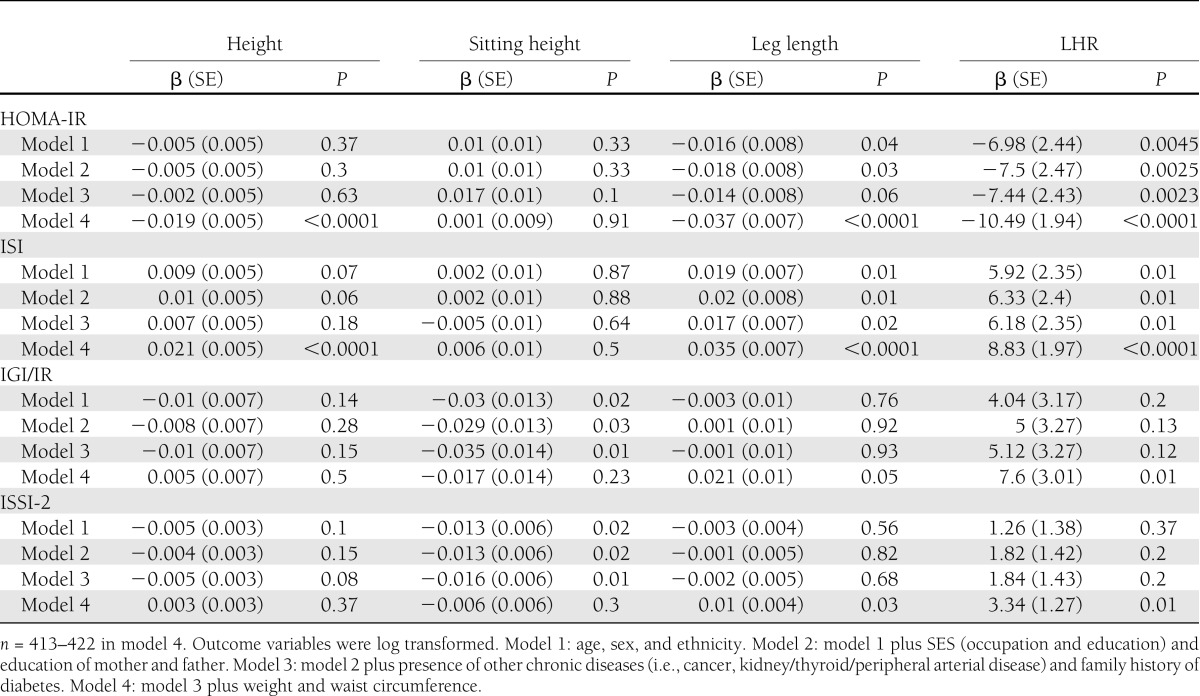
Multiple linear regression results are presented in Table 3. Significant inverse associations with HOMA-IR were found for leg length, LHR, and height after adjustment for all covariates in model 4 (β = −0.037 [SE 0.007], β = −10.49 [1.94], and β = −0.019 [0.005], respectively; all P < 0.0001). There were significant positive associations of leg length, LHR, and height with ISI after adjustment for model 4 covariates (β = 0.035 [0.007], β = 8.83 [1.97], and β = 0.021 [0.005], respectively; all P < 0.0001). Sitting height was not associated with HOMA-IR or ISI. In earlier models, LHR was not associated with IGI/IR or ISSI-2, but inclusion of model 4 covariates strengthened the association to the level of statistical significance (β = 7.6 [3.01], β = 3.34 [1.27], respectively; all P = 0.01). Similarly, leg length was also significantly positively associated with IGI/IR and ISSI-2 after adjustment for model 4 covariates (β = 0.021 [0.01], β = 0.01 [0.004], respectively; all P < 0.05). Initially, sitting height was significantly inversely associated with IGI/IR and ISSI-2 in models 1, 2, and 3 (β = −0.035 [0.014], β = −0.016 [0.006], respectively; all P = 0.01), but the significance was attenuated in models 4 (P > 0.23). Height was not significantly associated with ISSI-2 or IGI/IR.
Table 3.
Linear regression models showing associations of height, sitting height, leg length, and LHR with insulin sensitivity and β-cell function measures using the 3-year visit data from nondiabetic PROMISE subjects, adjusted for covariates
We conducted sensitivity analyses in which 1) stature component exposures were replaced by z scores of the corresponding variable, 2) leg length models were additionally adjusted by height, and 3) weight at 18 years was included in model 4. In each of these analyses, the results were not materially different from the primary analyses (data not shown).
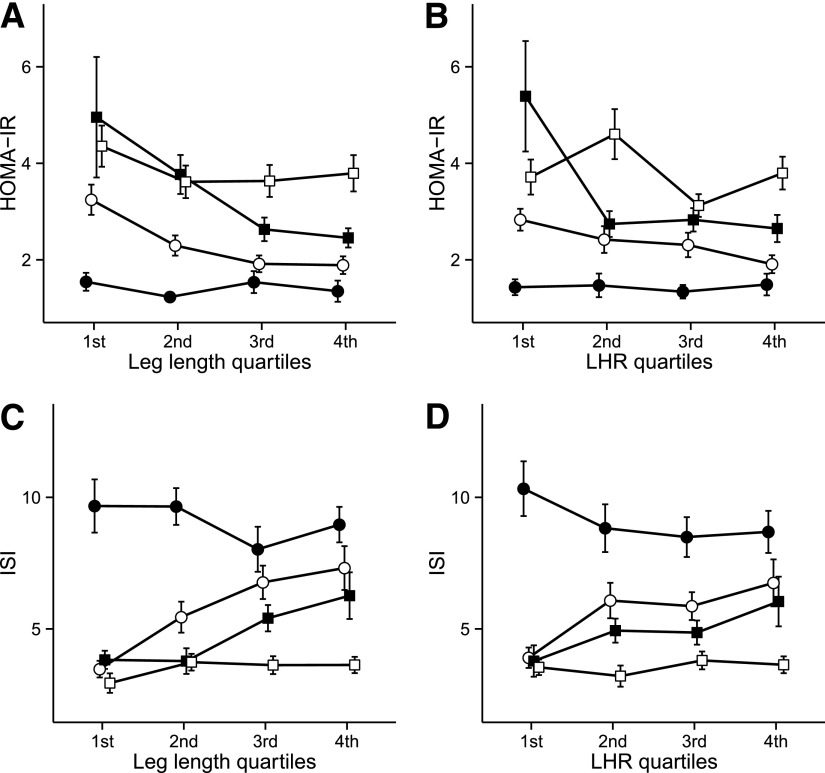
There were no significant interactions of sex, ethnicity, parental education, or weight on the associations of stature components with the outcome variables. However, there were significant interactions between waist circumference and leg length, height, and LHR on HOMA-IR (all P < 0.02) and ISI (all P < 0.025). Figure 1 illustrates the interactions of waist circumference with leg length and LHR on insulin sensitivity. Subjects in the first (60.0–88.3 cm) and fourth (108.1–141.0 cm) quartiles for waist circumference had the highest insulin sensitivity (HOMA-IR mean 1.4 [SEM 0.09], ISI 9.23 [0.48]) and lowest insulin sensitivity (HOMA-IR 3.75 [0.18], ISI 3.56 [0.16]), respectively, regardless of leg length. Among subjects with waist circumference in the 2nd and 3rd quartiles (88.4–108.0 cm), however, there was a significant modifying effect of leg length on the association of waist circumference with insulin sensitivity. For example, subjects in these two intermediate quartiles of waist circumference who had the shortest legs had insulin sensitivity levels approximating those of subjects with the largest waist (2nd quartile waist circumference HOMA-IR 3.25 [0.31], ISI 3.47 [0.32]). Third-quartile waist circumference was HOMA-IR 4.96 (1.25) and ISI: 3.83 (0.35); however, insulin sensitivity in these groups improved markedly as leg length increased (Fig. 1). The pattern of results for the interaction of waist circumference with height was similar to that of the waist circumference–leg length/LHR interaction (data not shown).
Figure 1.
Interaction of leg length on the association of waist circumference with measures of insulin sensitivity, adjusted for sex, ethnicity, and age. Points are means with SE bars. Lines within the plots depict quartiles of waist circumference. Leg length and LHR are in quartiles of HOMA-IR with leg length (A), HOMA-IR with LHR (B), ISI with leg length quartiles (C), and ISI with LHR (D). 1st quartile, ●; 2nd quartile, ○; 3rd quartile, ■; 4th quartile, □. Waist size ranges are, by quartile, 60.0–88.3 cm (1st), 88.4–98.9 cm (2nd), 99.0–108.0 cm (3rd), and 108.1–141.0 cm (4th). Leg length ranges are, by quartile, 63.6–73.7 cm (1st), 73.8–77.0 cm (2nd), 77.1–81.5 cm (3rd), and 81.6–94.5 cm (4th). LHR ranges are, by quartile, 0.430–0.457 (1st), 0.458–0.466 (2nd), 0.467–0.476 (3rd), and 0.477–0.516 (4th).
CONCLUSIONS
In the current study, we demonstrated that shorter leg length was independently associated with lower insulin sensitivity and β-cell function. In addition, we found a modifying effect of leg length on the association of waist circumference with measures of insulin sensitivity, indicating that increasing leg length partially mitigates the detrimental effect of increasing waist circumference on insulin sensitivity, though the association was not seen in individuals with the smallest or largest waist circumference.
Our findings regarding the inverse association of HOMA-IR with leg length are consistent with previous literature from an ethnically diverse U.S. population (12) and from several ethnically homogeneous male (20) and female (13,21) populations in Britain. The current study extends these findings with the use of a more detailed measure of insulin sensitivity. It has previously been shown (28) that HOMA-IR reflects hepatic insulin resistance in the postabsorptive, or fasted, state but contains little information on peripheral, or largely muscle, insulin sensitivity, which is predominant during the postprandial state. The ISI index used in the current study uses glucose and insulin levels after a glucose load and thus reflects whole-body insulin sensitivity rather than simply hepatic sensitivity. These findings are consistent with the thrifty phenotype hypothesis (2), which suggests that early-life insults during growth may lead to reduced insulin sensitivity (which allows for the redistribution of glucose during growth to higher priority organs), along with the underdevelopment of key organs.
β-Cell function measures have not previously been used in studies investigating the relationship of leg length with the metabolic disorders underlying type 2 diabetes. One previous study reported no association of height with ISSI-2 in pregnant females (34), although leg length was not measured. In the current study, we found that shorter legs were associated with lower β-cell function. This association may be mediated through the effects of early childhood developmental insults on the pancreas. Underdevelopment of the pancreas as a result of early life environmental insults may lead to reduced β-cell mass and cell number, a phenomenon that has been observed in mice born at a lower birth weight (35,36). Our findings suggest that in addition to fetal insults, early childhood insults may also decrease β-cell mass and thus the effective capacity of β-cells to secrete adequate insulin in response to glucose loads in later adulthood. The implications of this finding are that inadequate childhood conditions may impact the development of important organs and may have effects that persist into later adulthood.
Increasing sitting height was significantly associated with decreasing β-cell function (IGI/IR and ISSI-2) after adjusting for model 3 covariates. However, the association was attenuated after adjusting for weight and waist circumference. A possible explanation for this initial association is that longer sitting height increases the available area for deposition of abdominal visceral adipose tissue, which is more metabolically disruptive than subcutaneous adipose tissue. As both sitting height and weight were each inversely associated with β-cell function and sitting height was positively correlated with weight, not controlling for weight biased the estimate of sitting height with β-cell function away from the null; thus, our findings in models 1, 2, and 3 are of a significant association.
There were differences in the β-coefficients and significance between models 3 and 4 in the leg length and LHR with IGI/IR and ISSI-2 analyses. These differences were likely due to the strong influence that weight and waist circumference had on the associations. Since weight and waist circumference were positively correlated with leg length and were negatively correlated with the β-cell function variables, not controlling for weight and waist circumference biased the association of leg length with the outcome variables toward the null. There were also differences in the magnitude of the associations between the insulin sensitivity and the β-cell function outcomes. These differences may be due to the fact that β-cell function is difficult to accurately measure (while ISSI-2 and IGI/IR were validated against the disposition index, these correlations were moderate: r = 0.21–0.32) (30). The misclassification of these outcomes would have diluted the associations with our β-cell measures toward the null.
The interaction of leg length on the association of waist circumference with insulin sensitivity has potentially important ramifications. The results illustrated that those individuals in the 1st and 4th quartiles of waist circumference had the highest and lowest insulin sensitivity, respectively, regardless of leg length. For those individuals in the middle quartiles of waist circumference, however, leg length was positively associated with insulin sensitivity. Using waist circumference as a proxy measure of adulthood nutritional conditions, this interaction may illustrate the mismatch that may occur when the nutritional environment differs between early life and later adulthood, possibly leading to an increased risk for disease (2,22). Previous studies have found that those in the lowest category of birth weight (suggesting fetal insults) but in the highest category of adult BMI (suggesting adult overnutrition) had the greatest insulin resistance, measured by HOMA-IR (21). Our findings extend this birth weight and adult BMI observation by showing that adverse childhood conditions, through shorter legs, mismatched with an energetically replete adulthood, as manifested by a larger waist, may result in lower insulin sensitivity and thus may increase the risk for type 2 diabetes. However, a highly excessive energy imbalance in adulthood (i.e., the largest waist circumference) appears to offset any beneficial or harmful effect on insulin sensitivity that early childhood conditions may provide. By virtue of improving early childhood conditions, the health consequences of this early–late life mismatch may be ameliorated, thus possibly providing greater protection against developing type 2 diabetes in later adulthood.
Strengths of the current study include the comprehensive OGTT-derived measures for insulin sensitivity and β-cell function, which have not been used previously by other studies examining the association of leg length with type 2 diabetes traits. Use of these detailed measures allowed for a more comprehensive examination of the mechanisms involved in the relationship between early life deprivation and adult type 2 diabetes, examining both hepatic and whole-body insulin sensitivity and the capacity of the β-cells to respond to postprandial glucose loads. In addition, the subjects in this study were at risk for type 2 diabetes based on having one or more risk factors and thus represent a greater range in the metabolic capacity to dispose of glucose, which may be magnified by differences in early childhood conditions.
Limitations include the use of leg length as a marker of early life conditions and the observational nature of the study protocol. Optimal study designs to investigate the effects of early life conditions on later adult health require birth cohorts that are followed until late adulthood, with longitudinally measured outcomes and exposures such as diet, stress, SES, and disease biomarkers from blood samples. However, adult leg length is an established and accepted proxy measure of early childhood conditions and has been used in previous observational studies examining the impact of early childhood conditions (37–40). Various factors likely impact the growth of the legs, including childhood SES, nutrition, or stress, which have not been measured in the current study. Leg length measurements may also be prone to error resulting from vertebral abnormalities, such as vertebral fracture–induced kyphosis or scoliosis, which were not determined in the PROMISE cohort. Our study population consisted of subjects at risk for diabetes and may therefore not reflect the general population. However, we believe this sample of at-risk subjects is informative in the investigation of the natural history of diabetes because of the wide variability in metabolic abnormalities present in this sample. Previous studies have found an association between leg length and diabetes, as well as HOMA-IR, in the general population (12,13,20); our results extend these findings to individuals already displaying risk factors for developing diabetes. Finally, we were not able to adjust for birth weight (a potential confounder given the association of low birth weight with greater risk for diabetes) owing to a large number of missing or “unknown” responses (n = 107) to this question.
To summarize, shorter leg length was associated with lower insulin sensitivity and β-cell function. As leg length is a marker of early childhood conditions, the results from the current study suggest that environmental and nutritional insults during infancy and early life lead to decreased insulin sensitivity and a reduced capacity to secrete insulin in adulthood. Adults with shorter legs and an increasing waist circumference display a worsening metabolic profile, suggesting a health risk emerging from mismatched childhood–adulthood conditions. This study provides further evidence to support efforts to improve early life conditions to reduce risk for type 2 diabetes in adulthood.
Acknowledgments
This study was supported by grants from the Canadian Diabetes Association and the Connaught Fund from the University of Toronto. L.W.J. is supported by a Government of Ontario Graduate Scholarship. R.R. is supported by a Canadian Institutes of Health Research Clinical Research Initiative New Investigator Award, Canadian Diabetes Association Clinician-Scientist incentive funding, and a University of Toronto Banting and Best Diabetes Centre New Investigator Award. S.B.H. holds the Canadian Diabetes Association Chair in National Diabetes Management and the Ian McWhinney Chair of Family Medicine Studies at the University of Western Ontario. B.Z. holds the Sam and Judy Pencer Family Chair in Diabetes Research at Mount Sinai Hospital and University of Toronto. A.J.H. holds a Tier II Canada Research Chair in Diabetes Epidemiology.
No potential conflicts of interest relevant to this article were reported.
L.W.J. conducted the data analysis and wrote, reviewed, and edited the manuscript. S.B.H., R.R., H.C.G., and B.Z. researched data and reviewed and edited the manuscript. J.H. reviewed and edited the manuscript. A.J.H. researched data, contributed to the analysis and discussion, and reviewed and edited the manuscript. A.J.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
The authors thank the study subjects for their participation. The authors also thank Jan Neuman, Paula Van Nostrand, Stella Kink, and Annette Barnie of the Leadership Sinai Centre for Diabetes, Mount Sinai Hospital; and Sheila Porter and Mauricio Marin of the Centre for Studies in Family Medicine, University of Western Ontario, for their dedication and expert technical assistance.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJP. The thrifty phenotype hypothesis. Br Med Bull 2001;60:5–20 [DOI] [PubMed] [Google Scholar]
- 3.Lejarraga H. Growth in infancy and childhood: a pediatric approach. In Human Growth and Development. 1st ed. Cameron N, Ed. San Diego, Academic Press, 2002, p. 21–44 [Google Scholar]
- 4.Prokopec M. Differential rate of growth of the human body parts. In Perspectives in Human Growth, Development and Maturation. 1st ed. Dasgupta P, Hauspie R, Eds. Netherlands, Academic Press, 2001, p. 313–320 [Google Scholar]
- 5.Dangour AD, Schilg S, Hulse JA, Cole TJ. Sitting height and subischial leg length centile curves for boys and girls from Southeast England. Ann Hum Biol 2002;29:290–305 [DOI] [PubMed] [Google Scholar]
- 6.Li L, Dangour AD, Power C. Early life influences on adult leg and trunk length in the 1958 British birth cohort. Am J Hum Biol 2007;19:836–843 [DOI] [PubMed] [Google Scholar]
- 7.Wadsworth MEJ, Hardy RJ, Paul AA, Marshall SF, Cole TJ. Leg and trunk length at 43 years in relation to childhood health, diet and family circumstances; evidence from the 1946 national birth cohort. Int J Epidemiol 2002;31:383–390 [PubMed] [Google Scholar]
- 8.Schooling CM, Jiang CQ, Heys M, et al. Are height and leg length universal markers of childhood conditions? The Guangzhou Biobank cohort study. J Epidemiol Community Health 2008;62:607–614 [DOI] [PubMed] [Google Scholar]
- 9.Webb E, Kuh D, Peasey A, et al. Childhood socioeconomic circumstances and adult height and leg length in central and eastern Europe. J Epidemiol Community Health 2008;62:351–357 [DOI] [PubMed] [Google Scholar]
- 10.Clarkin PF. War, forced displacement and growth in Laotian adults. Ann Hum Biol 2012;39:36–45 [DOI] [PubMed] [Google Scholar]
- 11.Gunnell D, Smith GD, McConnachie A, Greenwood R, Upton M, Frankel S. Separating in-utero and postnatal influences on later disease. Lancet 1999;354:1526–1527 [DOI] [PubMed] [Google Scholar]
- 12.Asao K, Kao WHL, Baptiste-Roberts K, Bandeen-Roche K, Erlinger TP, Brancati FL. Short stature and the risk of adiposity, insulin resistance, and type 2 diabetes in middle age: the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. Diabetes Care 2006;29:1632–1637 [DOI] [PubMed] [Google Scholar]
- 13.Lawlor DA, Ebrahim S, Davey Smith G. The association between components of adult height and type II diabetes and insulin resistance: British Women’s Heart and Health Study. Diabetologia 2002;45:1097–1106 [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Tan H, Jeynes B. Is femur length the key height component in risk prediction of type 2 diabetes among adults? Diabetes Care 2009;32:739–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weitzman S, Wang C-H, Pankow JS, Schmidt MI, Brancati FL. Are measures of height and leg length related to incident diabetes mellitus? The ARIC (Atherosclerosis Risk in Communities) study. Acta Diabetol 2010;47:237–242 [DOI] [PubMed] [Google Scholar]
- 16.Han TS, Hooper JP, Morrison CE, Lean MEJ. Skeletal proportions and metabolic disorders in adults. Eur J Clin Nutr 1997;51:804–809 [DOI] [PubMed] [Google Scholar]
- 17.Schooling CM, Jiang C, Lam TH, et al. Height, its components, and cardiovascular risk among older Chinese: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Am J Public Health 2007;97:1834–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway BN, Shu X-O, Zhang X, et al. Age at menarche, the leg length to sitting height ratio, and risk of diabetes in middle-aged and elderly Chinese men and women. PLoS ONE 2012;7:e30625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langenberg C, Araneta MRG, Bergstrom J, Marmot M, Barrett-Connor E. Diabetes and coronary heart disease in Filipino-American women: role of growth and life-course socioeconomic factors. Diabetes Care 2007;30:535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith GD, Greenwood R, Gunnell D, Sweetnam P, Yarnell J, Elwood P. Leg length, insulin resistance, and coronary heart disease risk: the Caerphilly Study. J Epidemiol Community Health 2001;55:867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor DA, Davey Smith G, Ebrahim S. Life course influences on insulin resistance: findings from the British Women’s Heart and Health Study. Diabetes Care 2003;26:97–103 [DOI] [PubMed] [Google Scholar]
- 22.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol 2007;19:1–19 [DOI] [PubMed] [Google Scholar]
- 23.Hanley AJG, Retnakaran R, Qi Y, et al. Association of hematological parameters with insulin resistance and beta-cell dysfunction in nondiabetic subjects. J Clin Endocrinol Metab 2009;94:3824–3832 [DOI] [PubMed] [Google Scholar]
- 24.Kayaniyil S, Retnakaran R, Harris SB, et al. Prospective associations of vitamin D with β-cell function and glycemia: the PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study. Diabetes 2011;60:2947–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org, 1999 [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 27.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 28.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 2007;30:89–94 [DOI] [PubMed] [Google Scholar]
- 29.Wareham NJ, Phillips DI, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med 1995;12:931. [DOI] [PubMed] [Google Scholar]
- 30.Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med 2009;26:1198–1203 [DOI] [PubMed] [Google Scholar]
- 31.Wickham H. ggplot2: Elegant Graphics for Data Analysis New York, Springer, 2009
- 32.R Core Team. R: A language and environment for statistical computing [Internet], 2012. Vienna, Austria, R Foundation for Statistical Computing. Available from http://www.R-project.org Accessed May 2013
- 33.Kowall B, Rathmann W, Strassburger K, Meisinger C, Holle R, Mielck A. Socioeconomic status is not associated with type 2 diabetes incidence in an elderly population in Germany: KORA S4/F4 cohort study. J Epidemiol Community Health 2011;65:606–612 [DOI] [PubMed] [Google Scholar]
- 34.Kew S, Qi Y, Sermer M, et al. Relationship between short stature and postchallenge glycemia in pregnancy. Diabetes Care 2010;33:e173. [DOI] [PubMed] [Google Scholar]
- 35.Inoue T, Kido Y, Asahara S, et al. Effect of intrauterine undernutrition during late gestation on pancreatic beta cell mass. Biomed Res 2009;30:325–330 [DOI] [PubMed] [Google Scholar]
- 36.Hill DJ. Nutritional programming of pancreatic β-cell plasticity. World J Diabetes 2011;2:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaix B, Jouven X, Thomas F, et al. Why socially deprived populations have a faster resting heart rate: impact of behaviour, life course anthropometry, and biology—the RECORD Cohort Study. Soc Sci Med 2011;73:1543–1550 [DOI] [PubMed] [Google Scholar]
- 38.Gunnell D, Whitley E, Upton MN, McConnachie A, Smith GD, Watt GCM. Associations of height, leg length, and lung function with cardiovascular risk factors in the Midspan Family Study. J Epidemiol Community Health 2003;57:141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padez C, Varela-Silva MI, Bogin B. Height and relative leg length as indicators of the quality of the environment among Mozambican juveniles and adolescents. Am J Hum Biol 2009;21:200–209 [DOI] [PubMed] [Google Scholar]
- 40.Kim J-M, Stewart R, Shin I-S, Kim S-W, Yang S-J, Yoon J-S. Associations between head circumference, leg length and dementia in a Korean population. Int J Geriatr Psychiatry 2008;23:41–48 [DOI] [PubMed] [Google Scholar]