Table 1.
Hydrophenoxylation of unactivated internal alkynes.a
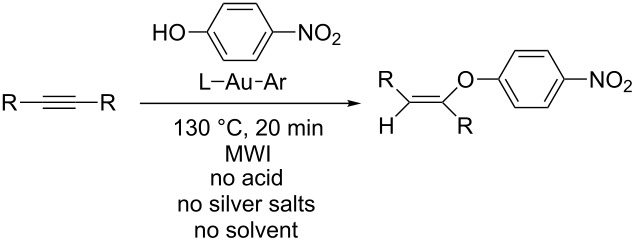 | |||
| Entry | Gold catalyst | Conv.b R = Ph Bu |
|
| 1 | None | 0 | 0 |
| 2 | (JohnPhos)AuCl | 0 | 0 |
| 3 | (t-BuXPhos)AuCl | 0 | 0 |
| 4 | (IMes)AuCl | 0 | 0 |
| 5 | (SIMes)AuCl | 0 | 0 |
| 6 | (IPr)AuCl | 0 | 0 |
| 7 | (SIPr)AuCl | 0 | 0 |
| 8 | 1 | 98 | 95 |
| 9 | 2 | 62 | 48 |
| 10 | 2 + t-BuXPhos | 79 | 60 |
| 11 | 3 | 77 | 70 |
| 12 | 4 | 47 | 50 |
| 13 | 5 | 63 | 48 |
| 14 | 6 | 64 | 45 |
| 15 | 7 | 86 | 92 |
| 16 | 8 | 89 | 95 |
| 17 | 9 | 90 | 91 |
| 18 | 1 Conventional heating | 95 | 93 |
aDiphenylacetylene or 5-decyne (0.28 mmol), 4-nitrophenol (0.56 mmol), catalyst (14.0 μmol, 5%), 130 °C, 20 min, no solvent, microwave irradiation. bBased upon 1H NMR spectroscopy using anisole as an internal standard.
