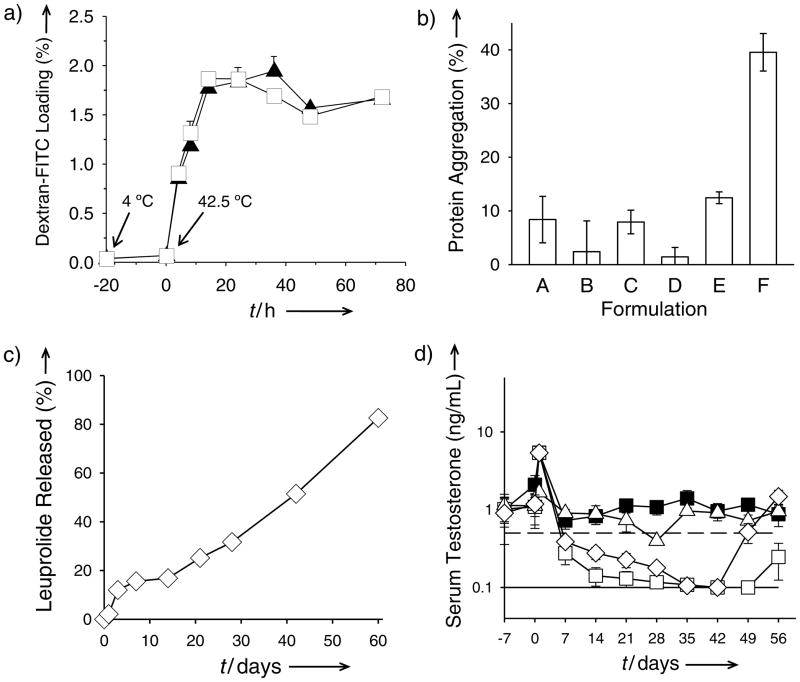
Figure 2.
PLGA self-healing microencapsulation (SM) minimizes biomacromolecule instability and provides long-term slow release in vivo. a) Kinetics of SM (initiation of self-healing at T > Tg). Effect of pre-hydration (incubation at 4 °C for 20 h and then at 42.5 °C (time = 0 h) with 65 mg/mL dextran-FITC) of microspheres (SM-3) on the SM of dextran-FITC. b) Insoluble aggregation after SM of encapsulation-labile lysozyme (Formulations A–D) and standard solvent-evaporation microencapsulation (Formulations E and F). Microspheres were prepared from 11 (A and B) and 51 (C–F) kDa Mw PLGA in the presence (B, D, F) and absence (A, C, E) of 0.45 M sucrose in the aqueous lysozyme solution (A and B: SM-4; C and D: SM-3; E and F: TM-1, Tables S1 and S2 in Supporting Information). c) and d) In vitro leuprolide acetate (LA) release characteristics (c) and in vivo serum testosterone suppression ability (d) of SM-microspheres (SM-5). Filled squares, open diamonds, open squares, and open triangles respectively correspond to soluble leuprolide, LA self-healing microencapsulated microspheres, commercial Lupron Depot, and SM-microspheres without LA. Solid and dashed lines respectively represent lower testosterone detection limit (0.1 ng/mL) and castration level (0.5 ng/mL). Values represent mean ± s.e.m.; n = 3 (a) or 5 (b and c) or 6 (d).