Abstract
Purpose
Define volumetric tumor growth rate in advanced NSCLC patients with sensitizing EGFR mutations initially treated with EGFR-TKI therapy beyond progression.
Methods
The study included 58 advanced NSCLC patients with sensitizing EGFR mutations treated with first-line gefitinib or erlotinib, who had baseline CT showing measurable lung lesion and at least two follow-up CTs while on TKI and experienced volumetric tumor growth. Tumor volume (mm3) of the dominant lung lesion was measured on baseline and follow-up CT scans during therapy. A total of 405 volume measurements were analyzed in a linear mixed effects model, fitting time as a random effect, to define the growth rate of the logarithm of tumor volume (logeV).
Results
A linear mixed effects model was fitted to predict growth of logeV, adjusting for time in months from baseline. LogeV was estimated as a function of time in months, in patients whose tumors have started growing after nadir: logeV=0.12*time+7.68 In this formula, the regression coefficient for time, 0.12/month, represents the growth rate of logeV (SE: 0.015; p<0.001). When adjusted for baseline volume, logeV0, the growth rate was also 0.12/month (SE: 0.015; p<0.001; logeV =0.12*months+0.72 logeV0+0.61).
Conclusion
Tumor volume models defined volumetric tumor growth after the nadir in EGFR-mutant advanced NSCLC patients receiving TKI, providing a reference value for the tumor growth rate in patients progressing after the nadir on TKI. The results can be further studied in additional cohorts to develop practical criteria which help to identify patients who are slowly progressing and can safely remain on EGFR-TKIs.
Keywords: lung cancer, computed tomography, tumor volume, tumor growth rate, EGFR tyrosine kinase inhibitors
INTRODUCTION
The characterization of genomic abnormalities in lung cancers in the past decade has transformed the way oncologists approach and treat lung cancer patients. This is best demonstrated by the discovery and clinical application of epidermal growth factor receptor (EGFR) mutation testing in non-small-cell lung cancer (NSCLC), which is associated with a dramatic radiographic response to EGFR tyrosine kinase inhibitors (TKI), gefitinib, erlotinib, and afatinib [1-3]. Patients with NSCLC harboring EGFR sensitizing mutations have the response rates greater than 70%, and progression-free survival of 9.7-13.1 months when treated with EGFR-TKIs [4-10]. However, virtually all patients with initial responses eventually progress due to the development of acquired resistance to EGFR-TKIs, and demonstrate radiographic tumor growth while on TKI [11-15].
Oncologists have used linear measurements defined by RECIST as a guide to define response and progression and determine when to switch therapy or add another agent [16-18]. However, recent clinical observations have indicated that the conventional RECIST-based assessment alone does not fully characterize response and progression in genomically-characterized patients with specific tumor types, including gastrointestinal stromal tumors (GIST), melanoma, lung cancer treated with targeted therapies [19-22]. New radiographic response criteria have been proposed, such as Choi criteria for GIST utilizing CT density, and immune-related response criteria (irRC) for melanoma in which response may be observed after initial increase of tumor burden [19-22].
Thoracic oncologists continue to treat NSCLC patients harboring EGFR mutations with EGFR-TKI beyond RECIST progression, because their tumors tend to grow slowly and patients remain asymptomatic, suggesting that some tumor cells remain sensitive to TKI [23-25]. Nishie et al demonstrated continuous EGFR-TKI after progression was associated with improved overall survival compared to those who switched to chemotherapy alone (HR: 0.42) [26]. EGFR-TKIs are associated with improved quality of life and less toxicity compared to chemotherapy [7-10]. The benefit of EGFR-TKI should be maximized by adequately prolonging the duration of TKI therapy. A previous study by our group examined NSCLC patients harboring EGFR mutations treated with first-line TKI, in which 88% of the patients continued TKI beyond RECIST progression, indicating that RECIST progression is not the single determining factor for terminating TKI [25]. There is a clear need for additional radiographic criteria of tumor growth beyond RECIST progression to better guide therapeutic decisions.
One of the major limitations of RECIST is the use of the cut-off value of tumor size increase to define progression, which does not incorporate the changes of tumor burden over time and the tumor growth rate. Tumor volume measurement using multidetector-row CT (MDCT) has been studied to complement limitations of RECIST. Tumor volume measurements in NSCLC are feasible with higher reproducibility than size measurements [27-31]. We have previously established a CT tumor volumetry technique in advanced NSCLC using FDA approved software [31]. The study showed tumor volume is more reproducible than size, which were consistent with other studies [27-31]. The volume assessment has also been used to predict outcome in NSCLC patients treated with chemotherapy and chest radiotherapy [32-34]. In advanced NSCLC patients with sensitizing EGFR mutations, tumor volume decrease at 8 weeks of EGFR-TKI therapy is associated with longer survival [35]. However, detailed characterization of volumetric tumor growth rate in EGFR-mutant NSCLC patients after initial response to EGFR-TKI therapy has not been systematically performed.
Tumor growth is based on a specific relationship between tumor volume and time, and comprehensive equations of tumor growth have been extensively pursued in the past decades. One of the well-studied models is the Gompertzian model, which was initially described by Gompertz in 1825 to deal with human mortality and later unexpectedly found useful to describe biological tumor growth [36-38]. Tumor growth by Gompertzian model has an exponential nature at the early stage, and subsequently saturates, approaching a plateau as tumor increases [37]. While the growth of most untreated tumors has been well described by the Gompertzian equation, the growth of treated tumors presents another investigational challenge [37-39]. In late 1970s, Looney et al quantitatively evaluated tumor growth curves in rat hepatoma during radiotherapy and chemotherapy, attempting to more precisely evaluate therapeutic effects, improve therapeutic scheduling, and better understand tumor biology [40-42]. These studies performed more than three decades ago, although technologically different, share similar concepts with the present study, in that they focused on tumor growth rate during therapy to improve response assessment and therapeutic decisions.
The purpose of the present study is to analyze the volumetric tumor growth rate in advanced NSCLC patients with sensitizing EGFR mutations after they reached their volume nadir during EGFR-TKI therapy, as an initial step to develop radiographic criteria for slow progression to aid therapeutic decision making.
METHODS
Patients
The study population included 58 patients with stage IV NSCLC (AJCC 7th edition) or stage I-IIIA NSCLC with systemic relapse and sensitizing EGFR mutations, treated with gefitinib or erlotinib as their initial systemic therapy for advanced NSCLC between February 2002 and May 2011 at Dana-Farber Cancer Institute. The patients had baseline CT demonstrating at least one measurable lung lesion (≥10mm in the longest diameter) and at least two follow-up CT scans during EGFR-TKI therapy, and experienced volumetric tumor growth while on TKI. Patients provided informed consent and their records were retrospectively reviewed with IRB approval.
Mutation analysis
Tumor specimens were obtained from diagnostic or surgical procedures. Samples consisted of frozen tumor specimens or paraffin embedded material. EGFR exons 18 to 21 were amplified by PCR and analyzed bidirectionally by direct sequencing for the presence of somatic mutations [1-2]. The following EGFR mutations were considered sensitizing: deletions, duplications, and deletion-insertions of exon19, L858R point mutation, L861Q point mutation, and G719 missense point mutations [25, 43-44].
Tumor volume measurements
Baseline and follow-up CT scans were performed to determine response to EGFR-TKI using the clinical chest CT protocol [31]. Follow-up CT scans were performed every 8 weeks for 33 patients enrolled in prospective trials of EGFR-TKI, and at the discretion of the treating providers for 25 patients treated off protocol [5,25,45-47]. A thoracic radiologist (M.N.) measured the volume of a dominant measurable lung lesion (one lesion/patient) on baseline and all CT during EGFR-TKI monotherapy, using FDA-approved volume analysis software previously validated (Vitrea ® 2, Vital Images, Minnetonka, MN)[31]. We utilized this technique based on our previously published data of high interobserver reproducibility, which demonstrated that the tumor volume measurements are more reproducible than size measurements [31]. The nadir (the smallest tumor volume recorded from baseline to TKI termination/last follow-up) was determined for each patient.
Statistical Analysis
A total of 405 volume measurements from the nadir to the end of TKI therapy/last follow-up, with data closure on 6/1/2012, were analyzed. Demographics and disease characteristics were summarized with descriptive statistics. A linear mixed effects model, fitting time as a random effect [48], was fitted to the repeated measures of volume data to estimate the effect of time and other prognostic factors on tumor growth. The tumor volume (mm3) was transformed to the natural logarithm scale, and the logarithm of tumor volume (logeV) was used. The first model was built adjusting only for time in months from baseline. Since the baseline volume (logeV0; the tumor volume measured on the baseline scan performed before the initiation of TKI therapy) may influence the tumor volume and its growth rate, the second model was adjusted for time and logeV0. The third model was adjusted for time, logeV0, and clinical characteristics shown in Table 1, to determine if clinical variables have significant effect on the tumor growth.
Table 1.
Patient and disease characteristics
| Variables | Number | % | |
|---|---|---|---|
| Sex | Female | 46 | 79.3 |
| Male | 12 | 20.7 | |
| Age | Median(range) | 62 | (35-84) |
| Race | White | 49 | 84.5 |
| Asian | 7 | 12.1 | |
| Black | 2 | 3.4 | |
| Smoking Status¶ | Never | 29 | 50.0 |
| Former | 28 | 48.3 | |
| Current | 1 | 1.7 | |
| Path | Adenocarcinoma | 53 | 91.4 |
| NSCLC NOS* | 5 | 8.6 | |
| 0 | 21 | 36.2 | |
| ECOG | 1 | 35 | 60.3 |
| Performance Status | 2 | 2 | 3.4 |
| Stage | I | 5 | 8.6 |
| II | 0 | 0 | |
| III | 0 | 0 | |
| IV | 53 | 91.4 | |
| TKI | Erlotinib | 53 | 91.4 |
| Gefitinib | 5 | 8.6 | |
| exon19 del | 35 | 60.3 | |
| exon19del & | |||
| EGFR mutations | L861Q | 1 | 1.7 |
| L858R | 16 | 27.6 | |
| L861Q | 1 | 1.7 | |
| L861Q & G719 | 1 | 1.7 | |
| G719 | 4 | 6.9 |
Never:<100 lifetime cigarettes; Former:quit ≥ 1 year prior to start of therapy; Current:smoked ≤1 year prior to start of therapy
NSCLC NOS, Non-small-cell lung cancer not otherwise specified
≫Extrathoracic metastasis at diagnosis of advanced disease
RESULTS
Patients
Table 1 summarizes the patient and disease characteristics. The median time on TKI monotherapy was 15.8 months. The median number of follow-up scans was 7.5 [range: 2-35]. The median time from baseline to tumor volume nadir was 6.3 months.
Volumetric tumor growth rate
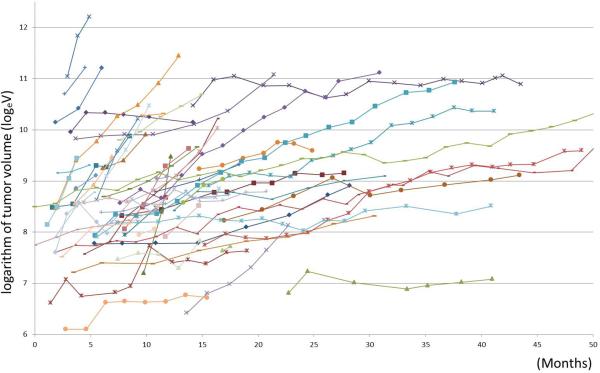
Figure 1 represents the volumetric tumor growth of 58 patients from their nadir to termination of therapy or the last follow-up scan. A linear mixed effects model was fitted to predict growth of logeV, adjusting for time from baseline.
Fig. 1.
Spiderplot represents volumetric tumor growth curves of the 58 patients after they reached their volume nadir.
In the first model, logeV was estimated as a function of time from the baseline, and the following equation was obtained:
In this equation, time represents the number of months from the baseline. The regression coefficient for time, 0.12/month, represents the growth rate of logeV (SE:0.015; p<0.001).
The second model was adjusted for logeV0 as a fixed effect, and logeV was estimated as follows:
LogeV0 was a significant predictor of the volume after the nadir (P<0.001), with the coefficient of 0.72. The growth rate of logeV, obtained as a coefficient for time, was 0.12/month (SE:0.015, P<0.001) after adjusting for logeV0. Therefore, the growth rate is 0.12/months for logeV, regardless of the baseline volume.
In the third model adjusted for the clinical variables and logeV0, stage at diagnosis (stage IV vs. others), TKI (gefitinib or erlotinib), and smoking status (current/former vs. never smoker) were significant predictors of the volume after the nadir, along with logeV0 (P<0.001 for logeV0, P=0.08 for stage, 0.04 for TKI, and 0.04 for smoking). The following equation was obtained:
(Stage=1 for stage IV, 0 for stage I-III; TKI=1 for gefitinib, 0 for erlotinib; smoking=1 for current/former smoker, 0 for never smoker). The growth rate of logeV was again 0.12/month (SE: 0.01, P<0.001). Stage, TKI and smoking affect how large the tumor volume is, however, the tumor growth rate is at 0.12/months for logeV, regardless of these clinical characteristics or baseline volume.
Threshold for volumetric tumor growth
To explore the appropriate criteria for tumor growth that can identify patients who can safely remain on EGFR-TKI, the threshold of the growth rate of logeV>0.15/months was proposed, based on the rate obtained from the equations (0.12/month) plus twice the standard error, 0.015 (0.12+0.015x2=0.15), representing the upper 95% confidence interval for the rate. We calculated the growth rate of logeV between 2 consecutive scans after nadir and investigated two consecutive occurrences of the growth rate of logeV>0.15/months during EGFR-TKI therapy in the 58 patients.
Fourteen patients (14/58, 24%) experienced the growth rate of logeV>0.15/months on two consecutive scans, which occurred after nadir in all patients (Figs 2-3). The median time from baseline to the second scan with rate >0.15/months was 9.7 months (range: 3.1-20.7). The median time on TKI in these 14 patients was 11.7 months, compared to 17.9 months in 44 patients who did not experience the rate >0.15/months on two consecutive scans. In 6 of the 14 patients (43%), TKI monotherapy was terminated within a month from the second scan, with no further CT before therapy termination. The remaining 8 patients (57%) remained on TKI monotherapy beyond the 2nd scan and had at least one additional chest CT scan while on TKI.
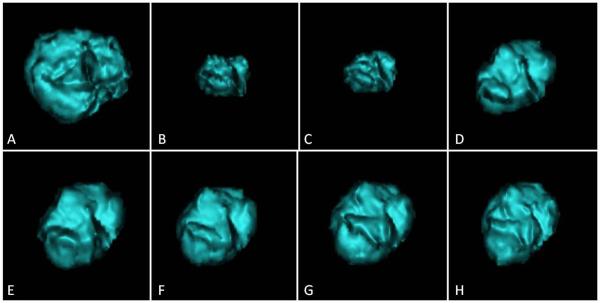
Fig. 2.
These are computed tomography (CT) images of a segmented lung tumor from a woman aged 35 years who had stage IV lung adenocarcinoma with fast tumor growth.
A. The baseline CT demonstrated a dominant mass in the right lower lobe, measuring 25,016 mm3.
B. The patient was treated with erlotinib. At 4 month of therapy, the tumor volume significantly decreased and reached the nadir, measuring 5,288 mm3.
C. At 5 month, the tumor started to grow back and measured 7,490 mm3. The growth rate of logeV since the prior scan was 0.22/month.
D. At 7 month, the tumor volume further increased, measuring 12,922 mm3. The growth rate of logeV since the prior scan was 0.33/month. A week later, erlotinib was discontinued and the patient was subsequently treated with platinum-based chemotherapy.
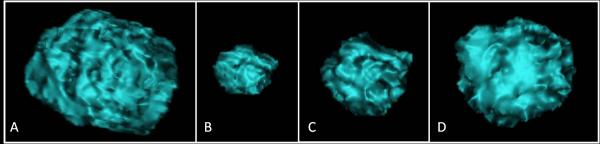
Fig. 3.
These are computed tomography (CT) images of a segmented lung tumor from a woman aged 52 years who had stage IV lung adenocarcinoma with slow tumor growth.
A. The baseline CT demonstrated a dominant right upper lobe lesion, measuring 14,495 mm3.
B. The patient was treated with gefitinib and tumor volume significantly decreased, reaching the nadir measuring 4,121 mm3 at 8 months.
C-H. The tumor started to grow back with gradual increase of tumor volume over a course of 2 years (C:11 month, D:16 month, E:19 month, F:21 month, G:26 months, H:28 month of therapy). The maximum growth rate of logeV between two consecutive scans since nadir was 0.09/month. Gefitinib was discontinued at 28 months and the patient was subsequently treated in a trial of irreversible EGFR inhibitor.
DISCUSSION
The present study provided the volumetric tumor growth rate after the nadir in EGFR-mutant advanced NSCLC patients receiving TKI. The result provides a reference value for the tumor growth rate in patients progressing on TKI, which can be further studied in additional cohorts and help to develop practical criteria that identify patients who can safely remain on EGFR-TKIs. To our knowledge, this is the first report providing a reference value of volumetric tumor growth in genomically-defined cohort of advanced NSCLC patients treated with targeted therapy.
The conventional RECIST-based assessment has limitations in characterizing tumor response and guiding therapeutic decisions in genomically-selected cohorts of patients receiving targeted therapy [19-24]. One of the major limitations of RECIST is that it does not take into account tumor growth dynamics, or tumor growth rate, which can be an important factor to characterize anti-cancer activity of targeting agents [49].
Characterizing the tumor growth rates has been a challenging topic for cancer researchers. Since 1970s, various studies attempted to characterize tumor growth dynamics of untreated and treated tumors, to better understand solid tumor biology and improve therapeutic management [37-42]. Recently, the concept of tumor growth rate during anti-cancer treatment was studied in trial patients with solid tumors, to define optimal trial endpoint [49-50]. Gomez-Roca et al studied 76 patients with solid tumors including NSCLC (n=21) treated in phase I trials, using the tumor volume estimated from tumor size. The tumor growth rate, obtained as log10(Vt/V0)/dt, decreased by 40% during treatment compared to the pretreatment, suggesting that integration of growth rate may improve assessment of treatment efficacy [50]. Others studied renal cell carcinoma patients and prostate cancer patients, and demonstrated that the growth rate constant, obtained as loge2/doubling time using tumor size, negatively correlated with overall survival [51-52].
These prior studies support that the tumor growth rate adds potentially useful information to assess response and predict outcome. The present study focused on EGFR-mutant NSCLC patients receiving TKI, since we believe that the tumor growth rate and its threshold for fast versus slow growth are cohort-specific and therapy-specific. We utilized the CT tumor volume measurements, rather than tumor size. We chose this approach since we have demonstrated that tumor volume measurement is more reproducible than size measurement and detects smaller changes more accurately [31]. In addition, the prior study by our group demonstrated that tumor volume decrease at 8 weeks of EGFR-TKI therapy was associated with longer survival in EGFR-mutant advanced NSCLC patients, while tumor size was not associated with survival [35].
Such approach in this highly specific genomically-defined cohort has never been performed to address the issue of tumor growth rate.
The initial model adjusting for time from baseline alone provided the growth rate of 0.12/months. The growth rate adjusting for time and baseline volume was also 0.12/month, indicating that the baseline volume does influence how big the tumor is at each time point after nadir, however, does not have much effect on how fast the tumor grows; the tumors in our cohort grow at an overall rate of 0.12/months for logeV after their nadir, regardless of their baseline volume. The result is consistent with our prior observation that baseline volume was not associated with survival in EGFR-mutant NSCLC patients receiving EGFR-TKI [35]. The growth rate was also 0.12/months adjusting for clinical variables, demonstrating the stability of the model. The model and the consistency of the growth rate of 0.12/month need to be validated in a larger independent cohort of patients with sensitizing EGFR mutations treated with TKI, in order to determine if the consistent results are due to an artifact of the model fitting or truly reflect biologically-driven behavior of EGFR-mutated tumor.
Our goal was to identify a cut-off value that can differentiate patients who are slowly progressing and can safely remain on EGFR-TKI. The upper limit of the 95%CI for the rate, 0.15/months was used because it is often used to determine outliers in the tumor volume studies and in growth models [51]. We investigated the occurrence of two consecutive observations of rate >0.15/months; only one observation can be a result of measurement variability rather than true tumor change, and clinicians tend to give a “benefit of a doubt” and confirm observations on one more scan before making decisions. The concept of confirmation is well-established in RECIST, and is given more emphasis in irRC, where confirmation is required for progression [16-19].
Two consecutive events of rate >0.15/months occurred after their nadir in all 14 patients, which is reassuring. The time on TKI was shorter in these 14 patients than in 44 patients without the events, indicating that the events did not happen by chance. The information about tumor growth rate was not available for the providers treating the patients. It is necessary to validate the model in an independent cohort to apply the threshold prospectively.
Limitations of the present study include a retrospective design, a small number of patients treated at a single institution. We are currently planning to expand the cohort and validate results in an independent cohort to establish practical criteria. The tumor volume was measured in one dominant lung lesion per patient, and smaller lung lesions or extrapulmonary lesions were not included, which is another limitation. We designed the study with this way since we believe tumor volume analysis should be additive to the evaluation of systemic tumor burden by RECIST-based approach and by clinical assessment [20, 35]. An ongoing multicenter phase II trial (NCT01310036; ASPIRATION study) allows continuation of erlotinib beyond RECIST-PD at investigator's discretion. Scenarios where erlotinib may be continued include PD after >6 months of PR/SD, asymptomatic minimal PD, or new brain metastasis controlled locally. Scenarios where erlotinib should be discontinued are symptomatic extracranial PD, rapid PD and/or worsening of performance status, or life-threatening complications [53]. The goal of our analysis is to provide a quantitative reference value that can be used along with these clinical criteria, to better aid therapeutic decisions and maximize the benefits of effective targeted therapy.
In conclusion, the tumor volume analysis was able to define volumetric tumor growth after the nadir in EGFR-mutant advanced NSCLC patients receiving EGFR-TKI. The study provided a reference value for the tumor growth rate in patients progressing after the nadir while they are on TKI. Further investigation is warranted to validate the results and develop practical radiographic criteria to help define patients as slow progressors who can safely remain on EGFR-TKIs.
The tumor volume analysis was able to define volumetric tumor growth after the nadir in EGFR-mutant advanced NSCLC patients receiving EGFR-TKI.
The goal of our tumor growth rate analysis is to provide a quantitative reference value that can be used along with these clinical criteria, to better aid therapeutic decisions of EGFR-mutant NSCLC patients and maximize the benefits of effective molecular targeted therapy.
Acknowledgments
The investigators were supported by 1K23CA157631(NCI)(M.N.), Grants 1RO1CA114465-07(B.E.J./P.A.J.) and 5R21 CA11627-02(H.H.) from the NIH, Grant No. 2P50CA090578-10(B.E.J./P.A.J.) from the NCI Specialized Program of Research Excellence in Lung Cancer, and a grant from Genentech Inc, as well as by the Doris and William Krupp Research Fund in Thoracic Oncology and ASCO Translational Research Professorship.
Footnotes
Disclosures:
Dr. Nishino has received support from an Eleanor Shore Fellowship and a Radiological Society of North America Research Scholar grant. Dr. Dahlberg has received salary support from Dr. Nishino’s NIH grant. Dr. Hatabu has received grants from Toshiba Medical Inc. and AZE Inc. Dr. Jackman has received compensation as a consultant to Genentech, Foundation Medicine, Inc., and Infinity Pharmaceuticals and has received fees for lectures from Chugai Pharma. Dr. Jänne has received compensation as a consultant and for drug development from Boehringer Ingelheim, Roche, Genentech, Abbott, AstraZenaca, Pfizer, and Sanofi and receives postmarketing royalties from LabCorp, a Dana-Farber Cancer Institute-owned intellectual property concerning EGFR mutations. Dr. Johnson has received research project (RO1) and SPORE grants from the NIH; has received compensation as a consultant from AstraZeneca and Genentech; serves on the advisory boards of Genentech and AstraZeneca; has received payment for a patent on EGFR mutation testing as an indication for EGFR-TKI therapy; and receives postmarketing royalties for EGFR mutation testing from Dana-Farber Cancer Institute for the licensed technology.
References
- 1.Paez JG, Janne PA, Lee JC, et al. EGFRmutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Zandwijk N, Mathy A, Boerrigter L, et al. EGFR and KRAS mutations as criteria for treatment with tyrosine kinase inhibitors: retro- and prospective observations in non-small-cell lung cancer. Ann Oncol. 2007;18:99–103. doi: 10.1093/annonc/mdl323. [DOI] [PubMed] [Google Scholar]
- 5.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–9. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 6.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–14. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 7.Mok TS, Wu YL. Thongprasert S: Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 8.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 10.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 11.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PloS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 14.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;23(3):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169–80. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Nishino M, Jagannathan JP, Ramaiya N, et al. Pictorial review of the new Response Evaluation Criteria in Solid Tumors: revised RECIST guideline version 1.1 – What oncologists want to know and what radiologists need to know. AJR. 2010;195:281–9. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 19.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 20.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized Tumor Response Assessment in the Era of Molecular Medicine: Cancer-specific and Therapy-specific Response Criteria to Complement Pitfalls of RECIST. AJR. 2012;198:737–45. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino M, Jackman DM, Hatabu H, Johnson BE, Van den Abbeele AD. Imaging of Lung Cancer in the Era of Molecular Medicine. Acad Radiol. 2011;18:424–36. doi: 10.1016/j.acra.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 23.Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5155. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 24.Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28:357–60. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishino M, Cardarella S, Dahlberg SE, et al. Radiographic Response Assessment & Therapeutic Treatment Decisions at the Time of RECIST Progression in EGFR-mutant NSCLC. Lung Cancer. 2013;79:283–8. doi: 10.1016/j.lungcan.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishie K, Kawaguchi T, Tamiya A, et al. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol. 2012;7:1722–7. doi: 10.1097/JTO.0b013e31826913f7. [DOI] [PubMed] [Google Scholar]
- 27.Zhao B, Schwartz LH, Moskowitz CS, et al. Lung cancer: computerized quantification of tumor response—initial results. Radiology. 2006;241:892–8. doi: 10.1148/radiol.2413051887. [DOI] [PubMed] [Google Scholar]
- 28.Zhao B, James LP, Moskowitz CS, et al. Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non-small cell lung cancer. Radiology. 2009;252:263–72. doi: 10.1148/radiol.2522081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozley PD, Schwartz LH, Bendtsen C, et al. Change in lung tumor volume as a biomarker of treatment response: a critical review of the evidence. Ann Oncol. 2010;21:1751–5. doi: 10.1093/annonc/mdq051. [DOI] [PubMed] [Google Scholar]
- 30.Mozley PD, Bendtsen C, Zhao B, et al. Measurement of Tumor Volumes Improves RECIST-Based Response Assessments in Advanced Lung Cancer. Transl Oncol. 2012;5:19–25. doi: 10.1593/tlo.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishino M, Guo M, Jackman DM, et al. CT Tumor Volume Measurement in Advanced Non-small-cell Lung Cancer: Performance Characteristics of Emerging Clinical Tool. Academic Radiology. 2011;18:54–62. doi: 10.1016/j.acra.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dehing-Oberije C, De Ruysscher D, van der Weide H, et al. Tumor volume combined with number of positive lymph node stations is a more important prognostic factor than TNM stage for survival of non-small-cell lung cancer patients treated with (chemo)radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1039–44. doi: 10.1016/j.ijrobp.2007.07.2323. [DOI] [PubMed] [Google Scholar]
- 33.Alexander BM, Othus M, Caglar HB, Allen AM. Tumor volume is a prognostic factor in non-small-cell lung cancer treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1381–7. doi: 10.1016/j.ijrobp.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 34.Kozak MM, Murphy JD, Schipper ML, et al. Tumor volume as a potential imaging-based risk-stratification factor in trimodality therapy for locally advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:920–6. doi: 10.1097/jto.0b013e31821517db. [DOI] [PubMed] [Google Scholar]
- 35.Nishino M, Dahlberg SE, Cardarella S, et al. Tumor volume decrease at 8 weeks predicts survival in EGFR-mutant advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor. Journal of Thoracic Oncology. 2013 Jun 19; doi: 10.1097/JTO.0b013e318294c909. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gompertz B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Phil. Trans. R. Soc.(Lond.) 1825;115:513. doi: 10.1098/rstb.2014.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro MA, Klamt F, Grieneisen VA, Grivicich I, Moreira JC. Gompertzian growth pattern correlated with phenotypic organization of colon carcinoma, malignant glioma and non-small cell lung carcinoma cell lines. Cell Prolif. 2003;36:65–73. doi: 10.1046/j.1365-2184.2003.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demicheli R, Foroni R, Ingrosso A, Pratesi G, Soranzo C, Tortoreto M. An exponential-Gompertzian description of LoVo cell tumor growth from in vivo and in vitro data. Cancer Res. 1989;49:6543–6. [PubMed] [Google Scholar]
- 39.Norton L, Simon R. Growth curve of an experimental solid tumor following radiotherapy. J Natl Cancer Inst. 1977;58:1735–41. doi: 10.1093/jnci/58.6.1735. [DOI] [PubMed] [Google Scholar]
- 40.Looney WB, Trefil JS, Schaffner JC, Kovacs CJ, Hopkins HA. Solid tumor models for the assessment of different treatment modalities: I. Radiation-induced changes in growth rate characteristics of a solid tumor model. Proc Natl Acad Sci U S A. 1975;72:2662–6. doi: 10.1073/pnas.72.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Looney WB, Trefil JS, Schaffner JG, Kovacs CJ, Hopkins HA. Solid tumor models for the assessment of different treatment modalities: systematics of response to radiotherapy and chemotherapy. Proc Natl Acad Sci USA. 1976;73:818–22. doi: 10.1073/pnas.73.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Looney WB, Trefil JS, Hopkins HA, Kovacs CJ, Ritenour R, Schaffner JG. Solid tumor models for assessment of different treatment modalities: therapeutic strategy for sequential chemotherapy with radiotherapy. Proc Natl Acad Sci U S A. 1977;74:1983–7. doi: 10.1073/pnas.74.5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–82. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–14. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackman DM, Cioffredi LA, Lindeman N, et al. Phase II trial of erlotinib in chemotherapy-naïve women with advanced pulmonary adenocarcinoma. J Clin Oncol. 2009;2715s(suppl) abstr 8065. [Google Scholar]
- 46.Jackman DM, Yeap BY, Lindeman NI, et al. Phase II clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:760–6. doi: 10.1200/JCO.2006.07.5754. [DOI] [PubMed] [Google Scholar]
- 47.Janne PA, Gurubhagavatula S, Yeap BY, et al. Outcomes of patients with advanced non-small cell lung cancer treated with gefitinib (ZD1839, “Iressa”) on an expanded access study. Lung Cancer. 2004;44:221–30. doi: 10.1016/j.lungcan.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 49.Levy A, Hollebecque A, Ferté C, et al. Tumor assessment criteria in phase I trials: beyond RECIST. J Clin Oncol. 2013;20(31):395. doi: 10.1200/JCO.2012.46.2184. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Roca C, Koscielny S, Ribrag V, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer. 2011;47:2512–2516. doi: 10.1016/j.ejca.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Stein WD, Wilkerson J, Kim ST, et al. Analyzing the pivotal trial that compared sunitinib and IFN-α in renal cell carcinoma, using a method that assesses tumor regression and growth. Clin Cancer Res. 2012;18:2374–81. doi: 10.1158/1078-0432.CCR-11-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–17. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park K, Tsai CM, Ahn M, et al. ASPIRATION: Phase II study of continued erlotinib beyond RECIST progression in Asian patients (pts) with epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). J Clin Oncol. 2012;(suppl) abstr TPS7614. [Google Scholar]