Abstract
Objective:
We tested the hypothesis that the order of exposure to maternal betamethasone and intra-amniotic (IA) lipopolysaccharide (LPS) will differentially modulate inflammation in the chorioamnion.
Study Design:
Time-mated Merino ewes with singleton fetuses received saline alone, IA LPS alone, maternal betamethasone before LPS, or betamethasone after LPS. We assessed inflammatory markers in the chorioamnion and the amniotic fluid.
Results:
Inflammatory cell infiltration, expression of myeloperoxidase, serum amyloid A3 (acute phase reactant) in the chorioamnion, and levels of interleukin (IL)-8 in the amniotic fluid increased 7 days after LPS exposure. Betamethasone prior to LPS decreased infiltration of the inflammatory cells, CD3+ T cells, and decreased the levels of IL-1β and IL-8 in the amniotic fluid.
Conclusions:
Betamethasone 7 days prior to LPS exposure suppressed LPS-induced inflammation. The markers of inflammation largely had returned to the baseline 14 days after LPS exposure.
Keywords: prematurity, preterm labor, innate immunity
Introduction
Premature birth continues to be a major national health concern, as it contributes disproportionately to neonatal morbidity and mortality, accounting for 12.3% of the live births and >60% of the neonatal deaths in the United States.1 Antenatal maternal corticosteroid treatment for women at risk of preterm birth is a standard clinical practice, and intramuscular betamethasone is preferred.2 A majority of very-low-birth-weight deliveries are exposed to infection or inflammation,3 which may be chronic and initiate preterm labor or develop after preterm labor or rupture of membranes. Intrauterine infection and inflammation are associated with adverse pulmonary, gastrointestinal, and neurological outcomes.4
Although there is a theoretical concern that maternal corticosteroids may augment chorioamnionitis, clinical studies and experimental evidence suggest that antenatal corticosteroids are also effective in the setting of chorioamnionitis.5–7 The delivery in many women given corticosteroids is delayed for several days to weeks,8 and histologic chorioamnionitis is clinically silent in a majority of women.4 Therefore, the combined exposures of antenatal corticosteroids and chorioamnionitis are common for the preterm fetus but the order of exposures can vary.
We previously reported that the simultaneous exposure of the preterm sheep fetus to antenatal corticosteroids and lipopolysaccharide (LPS) suppressed the LPS-induced pulmonary inflammation for 1 to 2 days after the exposure but amplified the inflammatory response to chorioamnionitis 5 to 15 days after the exposure.9,10 However, it is not known whether the order of exposure is important in mediating chorioamnionitis. Therefore, we evaluated the interactive effects of intra-amniotic (IA) LPS and antenatal corticosteroids on the severity of inflammation of the chorioamnion in fetal sheep.11 We tested the hypothesis that the order of exposure to maternal betamethasone and IA LPS will differentially impact inflammation of the chorioamnion. Fetal sheep were exposed in utero to IA LPS or antenatal maternal intramuscular (IM) betamethasone, with intervals of 7 days between the 2 interventions and the evaluation of chorioamnion inflammation.
Materials and Methods
The animal studies were performed in Western Australia, and The Institutional Animal Care and Use Committee (#8D05048) approved the protocol. Time-mated Merino ewes with singleton fetuses were randomly assigned to 1 of the 5 treatment groups or 1 control group (n = 5-8/group). The pulmonary outcomes and thymus development in these animals were previously reported.12,13 All ewes were treated with an IM injection of 150 mg of medroxyprogesterone acetate given on gestational day 100 to prevent preterm delivery induced by betamethasone in ewes.14 The ewes received IM injections of saline or betamethasone or an IA injection of LPS on day 107 and day 114 according to the group assignment (Table 1). Delivery was at 80% gestation at 121 ± 1 day. The doses administered were 0.5 mg/kg maternal weight β-methasone (Celestone Soluspan, Aspen pharmacare, New South Wales, Australia) once by IM injection either 7 days or 14 days prior to delivery and 10 mg LPS (Escherichia coli 055: B5; Sigma, St Louis, Missouri) by IA injection or an equivalent 2 mL volume of saline, either 7 days or 14 days prior to delivery. At delivery, the ewes were heavily anesthetized with an intravenous injection of ketamine (12 mg/kg) and medetomidine (0.12 mg/kg), followed by a subdural injection of 2% lignocaine hydrochloride (60 mg, 3 mL). The fetus was then delivered via surgical delivery. Fetal chorioamnion membranes were snap frozen for RNA analysis, and a roll was fixed in 10% buffered formalin (pH 7.4) for 24 hours for histological analysis. Amniotic fluid was snap frozen for measurement of cytokine proteins.
Table 1.
Study Groups and Treatments to the Ewe.a
| Group | N | Injection 1 107 days | Injection 2 114 days |
|---|---|---|---|
| Control | 5 | Saline (IM) | Saline (IA) |
| Sal/LPS | 6 | Saline (IM) | LPS (IA) |
| LPS/Sal | 6 | LPS (IA) | Saline (IM) |
| Sal/β | 7 | Sal (IA) | β (IM) |
| β/LPS | 6 | β (IM) | LPS (IA) |
| LPS/β | 6 | LPS (IA) | β (IM) |
Abbreviations: β, betamethasone; IA, intra-aminiotic; IM, intramuscular; LPS, lipopolysaccharide; Sal, saline.
a Delivery at 121 ± 1 days of gestation; (term gestation = 150 days).
Assessment of Chorioamnion Inflammation and Immunohistochemistry
Inflammatory cells in the chorioamnion were scored qualitatively and quantified by immunohistochemistry for markers of inflammatory cell activation using 5-µm paraffin sections from formalin-fixed chorioamnion. For qualitative scoring of chorioamnionitis, the sections were stained with hemotoxylin and eosin and staged and graded for degree of inflammation in a blinded manner using a modified Redline grading system.15 The scores for the magnitude and tissue distribution of neutrophil infiltration within the chorioamnion were reported as follows: score 0 = no chorioamnionitis, score 1 = stage 1, grade 1 chorioamnionitis, score 2 = stage 1, grade 2 chorioamnionitis, score 3 = stage 2, grade 2 chorioamniontis, and score 4 = stage 3, grade 2 chorioamnionitis.
Immunostaining for myeloperoxidase (MPO), monocyte chemotactic factor 1, and CD3 was performed after deparaffinization and rehydration of the fixed tissue, followed by antigen retrieval using citric acid buffer, pH 6.0 with microwave boiling, as reported.16 Endogenous peroxidase activity was blocked with methyl alcohol/hydrogen peroxide. Nonspecific interactions were inhibited with 2% goat serum during both primary and secondary antibody incubations. Sections were incubated with anti-MPO antibody (Cell marque, Rocklin, California; 1:500), a guinea pig polyclonal anti–monocyte chemotactic protein 1 (MCP-1; 1:1000) antibody (Seven Hill bioreagents, Cincinnati, Ohio), or a polyclonal rabbit antihuman T-cell (CD3) antibody (DakoCytomation, Carpinteria, California; 1:100). Following the overnight incubation with the primary antibody at 4°C, the sections were incubated with the appropriate secondary antibody for 30 minutes at room temperature (1:200). Immunostaining was visualized using a Vectastain ABC peroxidase Elite kit to detect the antigen–antibody complexes (Vector Laboratories Inc, Burlingame, California). Antigen detection was enhanced with nickel diaminobenzidine, followed by incubation with TRIS cobalt to give a black precipitate. Nuclei were counterstained with nuclear fast red for photomicroscopy. Blind scoring of inflammation in the chorioamnion was done by counting MCP-1, MPO, or CD3 positive cells in 10 comparable nonoverlapping high-power fields of each animal (5-6 animals/group). The average cell count per animal was used to compute the group averages and standard error of mean.
Cytokine Messenger RNA Quantitation
Total RNA was isolated from the frozen chorioamnion after homogenization in TRIzol reagent (Invitrogen, Carlsbad, California) using a modified Chomczynski method.9 Reverse transcriptase was performed using Verso cDNA Kit (Thermo Scientific, Waltham, Massachusetts) to produce a single-strand complementary DNA (cDNA). The genes interleukin (IL)-1β, IL-6, IL-8, IL1-RA, MCP-1, and serum amyloid A3 (SAA3) were amplified using the cDNA template and sheep-specific primers along with Taqman probes (Applied Biosystems, Carlsbad, California) in a real-time polymerase chain reaction assay (Applied Biosystems, Foster City, California). The messenger RNA (mRNA) expression for each gene was normalized to the mRNA for the ribosomal protein 18S as an internal standard. Final data were expressed as fold increase over the control value, which was assigned a value of 1.
Cytokine Analysis in the Amniotic Fluid
Cytokine protein quantifications were performed using sandwich enzyme-linked immunosorbent assay (ELISA) assays as described.17 The following antibody sets were used: for IL-1b (coating antibody—rabbit antiovine IL-1b and primary antibody—guinea pig antiovine IL-1b [Seven Hills Bioreagents]), IL-6 (coating antibody—mouse anti-ovine IL-6 [Chemicon # MAB1004] and primary antibody—rabbit anti-ovine IL-6 [Chemicon # AB1839]), and IL-8 (coating antibody—mouse anti-ovine IL-8 [Chemicon # MAB10445] and primary antibody—rabbit anti-ovine IL-8 [Chemicon # AB1840]). The detection antibody in all the assays was an appropriate species-specific horse radish peroxidase–conjugated antibody. The lowest detection limits and dynamic ranges were IL-1β and IL-6 (0.195-12.5 ng/mL), IL-8 (0.39-25 ng/mL), and MCP-1 (0.1-80 ng/mL). The correlation coefficients were .94 to .99 for all the assays.
Data Analysis
Results are given as mean ± standard error. Comparisons between the 5 groups were performed by 1-way analyses of variance using the nonparametric Kruskal-Wallis test. To determine the differences between the 2 groups, a nonparametric t test (Mann-Whitney U test) was used, since the data were not normally distributed. Statistical significance was accepted at P < .05.
Results
Assessment of Inflammation in the Chorioamnion
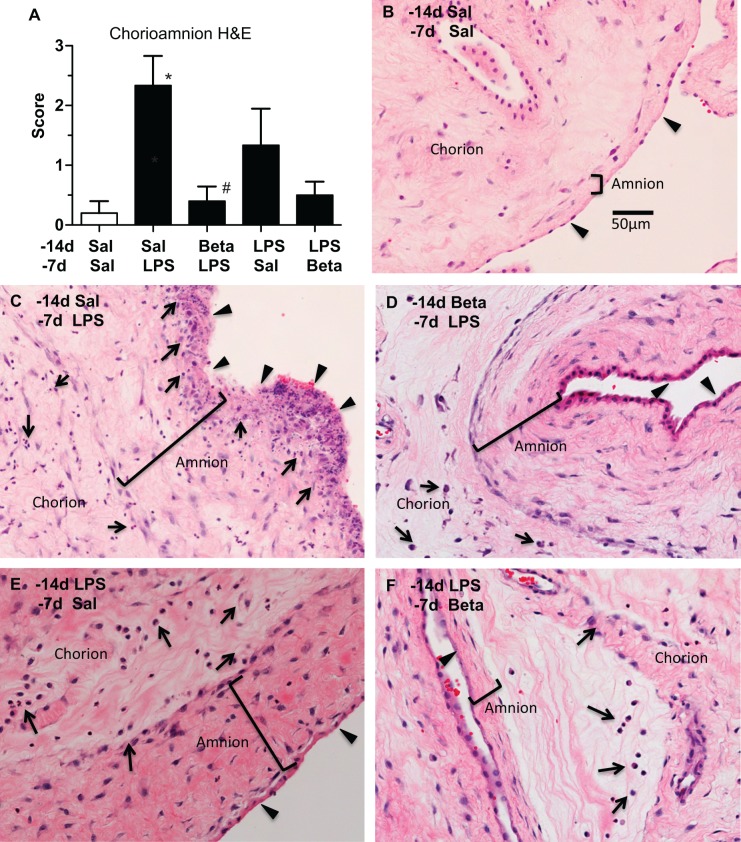
The inflammation scores were different between the groups (P = .02; Figure 1A). As expected, the control group (Sal/Sal) had minimal inflammation. Compared to controls, the exposure to LPS 7 days before delivery (Sal/LPS) or LPS 14 days before delivery (LPS/Sal) increased the inflammatory scores. Exposure to LPS disrupted the amniotic epithelium with necrosis of the epithelial layer, thickened the amnion, and caused infiltration of neutrophils in both the chorion and the amnion (Figure 1C), in some animals. Interestingly, compared to LPS exposure alone, maternal β-methasone prior to LPS exposure (Beta/LPS group) had minimal inflammation, with neutrophil infiltration limited to the chorion and sparing of the amnion epithelium. However, the subepithelial amnion mesenchyme was thickened (Figure 1D). Compared to the 14-day LPS alone (LPS/Sal), treatment with β-methasone after LPS (LPS/Beta) also decreased the inflammation, limited the neutrophils invasion to the chorion, and the subepithelial amnion was no longer thickened (Figure 1F). The magnitude of suppression of inflammation was greater when β-methasone was given before LPS (Beta/LPS) compared to β-methasone given after LPS (LPS/ Beta).
Figure 1.
Maternal β-methasone (β) prior to intra-amniotic lipopolysaccaride (LPS) blunts histologic chorioamnionitis (A). Scores indicating severity of histologic chorioamnionitis are shown for the different groups. Representative photomicrographs of hematoxylin and eosin staining are shown for (B) Saline 14 days/Saline 7 days (control), (C) Saline14 days/LPS 7 days, (D) Beta 14 days/LPS 7 days, (E) LPS 14 days/Saline 7 days, and (F) LPS 14 days/β 7 days groups. Arrows indicate inflammatory cells and arrow heads identify the amniotic epithelium. Note the thickened amnion and sloughing of amniotic epithelium in (C). N = 4-6 animals/group, scale bar (B) represents 50 µm, *P < .05 versus saline 14 days/saline 7 days, #P < .05 versus Saline14 days/LPS 7 days.
Immunohistology for Characterization of Inflammatory Cells in the Chorioamnion
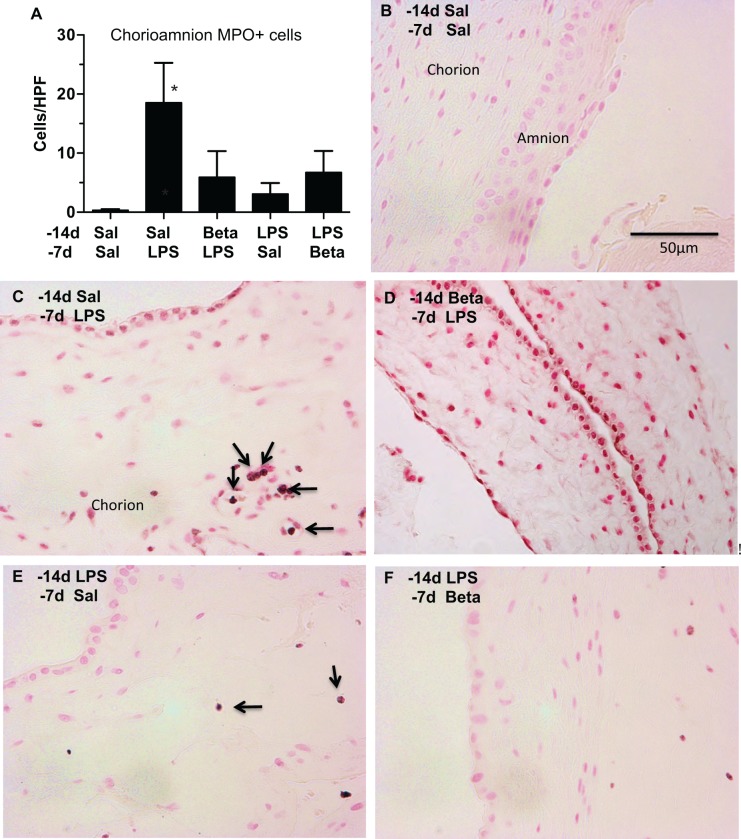
Compared to the controls, the numbers of MPO-expressing cells were significantly elevated 7 days after LPS exposure (Sal/LPS; Figure 2). β-methasone 7 days prior to LPS exposure (Beta/LPS) qualitatively decreased the number of MPO-expressing cells (compare Figure 2 panels C and D). After 14 days of LPS exposure (Sal/LPS), the MPO-expressing cell numbers were no different compared to the control group. β-methasone after LPS (LPS/Beta) did not significantly alter the number of MPO-positive cells.
Figure 2.
Myeloperoxidase (MPO) expression in inflammatory cells after maternal β-methasone (β) and intra-amniotic lipopolysaccharide (LPS; A). Mean MPO-expressing cells per microscopic field are shown for the different groups. Representative photomicrographs of MPO immunostaining are shown for (B) Saline 14 days/Saline 7 days (control), (C) Saline14 days/LPS 7 days, (D) β 14 days/LPS 7 days, (E) LPS 14 days/Saline 7 days, and (F) LPS 14 days/β 7 days groups. Arrows point to MPO-expressing cells. N = 4-6 animals/group, scale bar (B) represents 50 µm, *P < .05 versus Saline 14 days/Saline 7 days.
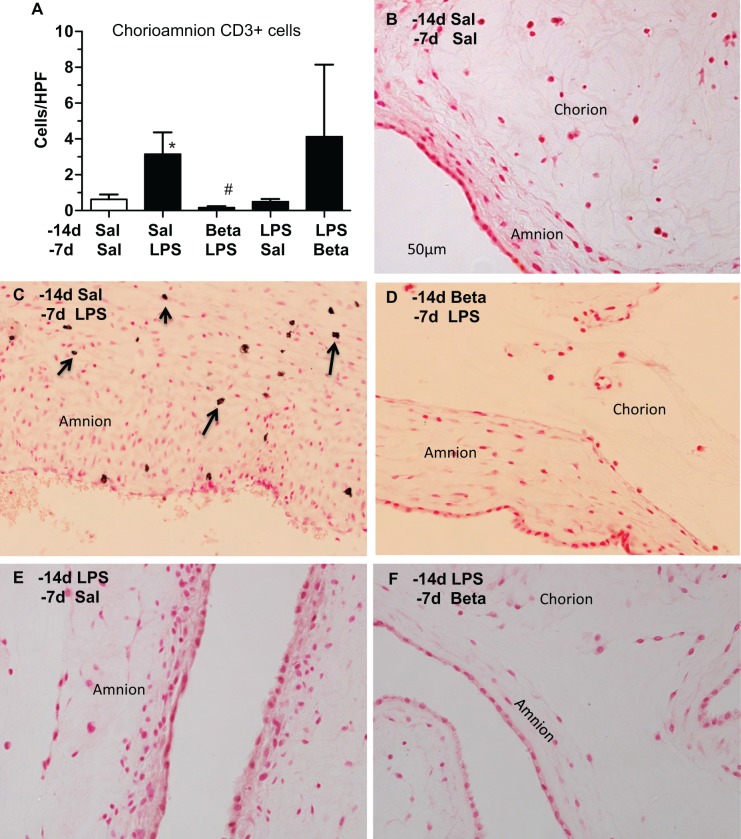
The number of T cells expressing CD3 in the chorioamnion increased 7 days after LPS exposure (Figure 3). Compared to LPS exposure alone, there was a decrease in CD3-expressing T cells when β-methasone was given prior to LPS. The T-cell infiltration was no longer apparent 14 days after LPS exposure. In the animals given β-methasone followed by LPS (Beta/LPS), only 1 of the 4 had CD3+ T-cell infiltration.
Figure 3.
Maternal β-methasone (β) prior to intra-amniotic lipopolysaccharide (LPS) blunts infiltration of CD3+ T cells in the chorioamnion (A). Mean CD3-expressing cells per microscopic field are shown for the different groups. Representative photomicrographs of CD3 immunostaining are shown for (B) Saline 14 days/Saline 7 days (control), (C) Saline14 days/LPS 7 days, (D) β 14 days/LPS 7 days, (E) LPS 14 days/Saline 7 days, and (F) LPS 14 days/β 7 days groups. Arrows point to CD3-expressing cells in (C). N = 4 animals/group, scale bar (B) represents 50 µm, *P < .05 versus Saline 14 days/Saline 7 days, #P < .05 versus Saline14 days/LPS 7 days.
Cytokine mRNAs in the Chorioamnion
Consistent with our previous data demonstrating short-lived increases in cytokine mRNAs (up to 4 days) in the chorioamnion after LPS exposure,11 IL-1b, IL-1ra, IL-6, and IL-8 levels in the chorioamnion were not significantly different between the control and the treatment groups (Table 2). Compared to controls, the expression of the acute phase reactant SAA3 mRNA was significantly increased 7 days but not 14 days after LPS exposure (Table 2). Exposure to β-methasone prior to LPS exposure significantly decreased LPS-induced SAA3 mRNA expression.
Table 2.
Quantitation of Cytokine Messenger RNA in the Chorioamnion.a
| Groups | IL-1β | IL-6 | IL-8 | MCP-1 | SAA3 | IL-1ra |
|---|---|---|---|---|---|---|
| Sal/Sal | 1 ± 0.2 | 1 ± 0.2 | 1 ± 0.9 | 1 ± 0.2 | 1 ± 0.4 | 1 ± 0.4 |
| Sal/LPS | 8.5 ± 5.0 | 3.6 ± 2.2 | 5.6 ± 3.8 | 4.9 ± 2.7 | 140 ± 125b | 1.2 ± 0.4 |
| β/LPS | 0.8 ± 0.4 | 0.7 ± 0.4 | 0.1 ± 0.04c | 0.9 ± 0.3 | 7.9 ± 7.5 | 0.5 ± 0.1 |
| LPS/Sal | 10.2 ± 2.8b | 3.9 ± 2.2 | 1.2 ± 0.4 | 9.6 ± 4.5 | 24.7 ± 11.9 | 0.6 ± 0.2 |
| LPS/β | 16.2 ± 14.8 | 1.3 ± 0.7 | 4 ± 3.6 | 2.5 ± 1.0 | 8.6 ± 6.6 | 1.2 ± 0.4 |
Abbreviations: Sal, intra-amniotic saline; β, maternal intramuscular β-methasone; LPS, lipopolysachharide; SAA3, serum amyloid A3; SEM, standard error of the mean.
a Data expressed as mean ± SEM.
b P < .05 versus Sal/Sal (control).
c P < .05 versus Sal/LPS.
Cytokines in the Amniotic Fluid
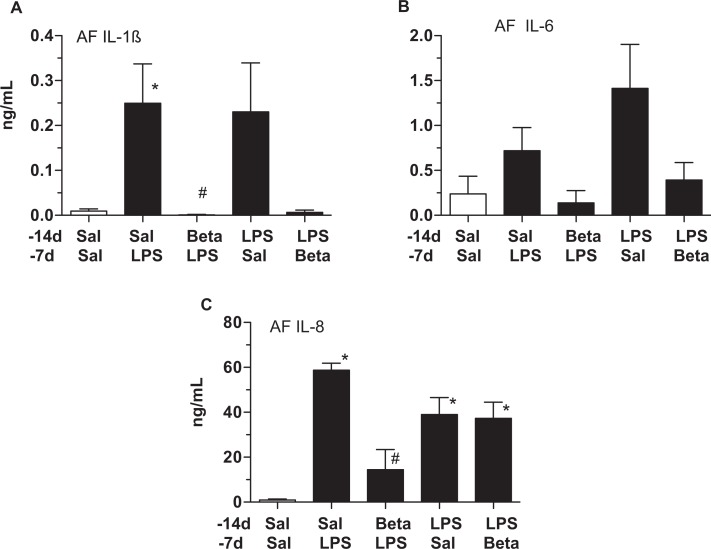
Compared to controls, IL-1β and IL-8 levels increased in the amniotic fluid 7 days after LPS exposure (Figure 4A and 4C). β-methasone suppressed both the IL-1β and the IL-8 increases, prior to LPS exposure. Although IL-6 levels also increased after LPS exposure, these changes were not statistically significant (Figure 4B). The 14-day LPS exposure group had increased IL-8 levels compared to controls, while IL-1β increases were variable within the 14-day LPS-exposure group. In contrast to β-methasone prior to LPS exposure (Beta/LPS), β-methasone after LPS exposure (LPS/Beta) did not suppress IL-8 induction (Figure 4C).
Figure 4.
Maternal β-methasone (β) prior to intra-amniotic lipopolysaccharide (LPS) blunts expression of proinflammatory cytokines/chemokines in the amniotic fluid. Amniotic fluid cytokines were quantitated using enzyme-linked immunosorbent assay (ELISA). A, IL-1β, (B) IL-6, and (C) IL-8. Compared to the saline 14 days/LPS group, β 14 days/LPS 7 days animals had lower IL-1β and IL-8 levels. N = 4-6 animals/group, *P < .05 versus saline 14 days/saline 7 days, # P < .05 versus saline14 days/LPS 7 days.
Discussion
Antenatal glucocorticoids are routinely given to women with histologic chorioamnionitis and to women at risk of preterm delivery.5–7 Since chorioamnionitis is clinically silent in a large number of women, the relative timing of β-methasone and the onset of chorioamnionitis is not known.18 Despite the common occurrence, surprisingly little is known about the interactions between the 2 exposures, each of which have pleotropic effects. Our results demonstrate that administration of maternal β-methasone prior to exposure to LPS decreased the severity of chorioamnionitis. Both infiltration of inflammatory cells and activation decreased when β-methasone was given 14 days before delivery followed by LPS exposure 7 days before delivery. Although most indicators of chorioamnionitis returned to control levels 14 days after IA LPS, amniotic fluid IL-8 levels remained persistently elevated. Interestingly, maternal β-methasone 7 days after LPS exposure did not reduce the amniotic fluid IL-8 levels.
Inflammation during chorioamnionitis requires both infiltration of leukocytes in the tissue and activation of the inflammatory cells. Neutrophil, monocyte, and T-cell infiltration in the chorioamnion were evident 7 days after LPS exposure with less infiltration 14 days after IA LPS. The inflammatory cells were activated because they expressed MPO, and the cytokines IL-1β and IL-8 were increased in the amniotic fluid. Although IL-6 levels in the amniotic fluid have been used to diagnose chorioamnionitis in humans, we have previously reported that fetal IL-6 expression is not prominent after IA LPS exposure in the sheep.11 Consistent with our previous studies, IL-6 levels in the amniotic fluid were variable in this study. Similarly, the mRNA expression for cytokines was not significantly increased in the chorioamnion 7 or 14 days after LPS exposure, consistent with the previous reports of mRNA induction lasting 4 days after IA LPS.11
The relative timing of maternal β-methasone and IA LPS is important in the modulation of chorioamnionitis. We previously reported that when ewes were simultaneously given IA LPS and IM β-methasone to LPS, the chorioamnion inflammation was suppressed for 1 to 2 days after exposures, but the suppression was no longer evident 5 to 15 days after the exposures.19 Maternal β-methasone and IA LPS have different pharmacokinetic profiles. In fetal sheep, maternal β-methasone treatment resulted in peak fetal β-methasone levels 3 hours after treatment with a decrease to 50% of the peak levels at 6 hours.20 In contrast, the half-life of endotoxin in the amniotic fluid was approximately 30 hours in the sheep.19 Fetal β-methasone levels 7 days after maternal treatment will be negligible. Therefore, the inhibition of LPS-induced chorioamniontis 7 days after maternal β-methasone exposure suggests priming or conditioning of the inflammatory response cells rather than a drug–drug interaction.
The modulation of LPS-induced inflammation in the chorioamnion by β-methasone is consistent with the results reported by our group in other fetal organs in the same animals. β-methasone administered 7 days before IA LPS suppressed fetal lung inflammation when assessed 7 days after IA LPS injection.13 Interestingly, LPS-induced lung inflammation 14 days after injection was not inhibited by maternal β-methasone 7 days after LPS exposure.13 Lung volumes, a proxy for lung maturation, were higher in the 14-day LPS + 7-day beta animals compared to 14-day Beta+ 7-day LPS animals, but both the groups had higher lung volumes than those exposed to β-methasone alone.13 In the same animals, IA LPS induced involution of the thymus and activation of the fetal thymocytes.12 β-methasone exposure prior to LPS exposure largely suppressed LPS effects on the fetal thymus, while β-methasone after IA LPS injection had no significant effects. These results demonstrate that maternal β-methasone modulates the effects of chorioamnionitis in multiple maternal–fetal compartments.
There are some limitations in this study. From a clinical perspective, a common scenario is administration of β-methasone in women with preterm rupture of membranes, a surrogate marker for histologic chorioamnionitis. We could not adequately address the clinical scenario of exposure to β-methasone after chorioamnionitis, because chorioamnion inflammation had largely subsided 14 days after exposure to LPS. Nevertheless, amniotic fluid IL-8 did not decrease when β-methasone was given 7 days after IA LPS, suggesting a lack of immunomodulation by β-methasone when given after LPS exposure. Whether β-methasone has an immunomodulatory role in chorioamnionitis induced by live microorganisms as opposed to LPS needs to be studied. Finally, immunomodulation of different time intervals between inflammatory stimuli and β-methasone, as will happen clinically, needs to be explored.
Our experiments do not identify new concerns for the use of antenatal corticosteroids in women. Both IA LPS and maternal β-methasone improve lung compliance in sheep fetuses and the combination further improves lung compliance.13,21 The observation that β-methasone can suppress LPS-induced chorioamnionitis if β-methasone exposure precedes IA LPS injection has clinical implications.
Acknowledgments
We thank Professor Jane Pillow, Dr Ilias Nitsos, and Dr Graeme Polglase for helping with animal use.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funded by NIH grant HD57869 (to SGK).
References
- 1. Mathews TJ, Minino AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics. 2011;127(1):146–157. [DOI] [PubMed] [Google Scholar]
- 2. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. [DOI] [PubMed] [Google Scholar]
- 3. DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–808. [DOI] [PubMed] [Google Scholar]
- 5. Harding JE, Pang J, Knight DB, Liggins GC. Do antenatal corticosteroids help in the setting of preterm rupture of membranes? Am J Obstet Gynecol. 2001;184(2):131–139. [DOI] [PubMed] [Google Scholar]
- 6. Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol. 2006;195(4):1020–1024. [DOI] [PubMed] [Google Scholar]
- 7. Been JV, Degraeuwe PL, Kramer BW, Zimmermann LJ. Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis. BJOG. 2011;118(2):113–122. [DOI] [PubMed] [Google Scholar]
- 8. Banks BA, Cnaan A, Morgan MA, et al. Multiple courses of antenatal corticosteroids and outcome of premature neonates. North American Thyrotropin-Releasing Hormone Study Group. Am J Obstet Gynecol. 1999;181(3):709–717. [DOI] [PubMed] [Google Scholar]
- 9. Kallapur SG, Kramer BW, Moss TJ, et al. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2003;284(4):L633–L642. [DOI] [PubMed] [Google Scholar]
- 10. Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res. 2004;55(5):764–768. [DOI] [PubMed] [Google Scholar]
- 11. Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;280(3):L527–L536. [DOI] [PubMed] [Google Scholar]
- 12. Kuypers E, Collins JJ, Jellema RK, et al. Ovine fetal thymus response to lipopolysaccharide-induced chorioamnionitis and antenatal corticosteroids. PLoS One. 2012;7(5):e38257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuypers E, Collins JJ, Kramer BW, et al. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2012;302(4):L380–L389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jobe AH, Newnham JP, Moss TJ, Ikegami M. Differential effects of maternal betamethasone and cortisol on lung maturation and growth in fetal sheep. Am J Obstet Gynecol. 2003;188(1):22–28. [DOI] [PubMed] [Google Scholar]
- 15. Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435–448. [DOI] [PubMed] [Google Scholar]
- 16. Berry CA, Nitsos I, Hillman NH, et al. Interleukin 1 in Lipopolysaccharide Induced Chorioamnionitis in the Fetal Sheep. Reprod Sci. 2011;18(11):1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah TA, Hillman NH, Nitsos I, et al. Pulmonary and systemic expression of monocyte chemotactic proteins in preterm sheep fetuses exposed to lipopolysaccharide-induced chorioamnionitis. Pediatr Res. 2010;68(3):210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Eng J Med. 2000;342(20):1500–1507. [DOI] [PubMed] [Google Scholar]
- 19. Newnham JP, Kallapur SG, Kramer BW, et al. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am J Obstet Gynecol. 2003;189(5):1458–1466. [DOI] [PubMed] [Google Scholar]
- 20. Berry LM, Polk DH, Ikegami M, Jobe AH, Padbury JF, Ervin MG. Preterm newborn lamb renal and cardiovascular responses after fetal or maternal antenatal betamethasone. Am J Physiol. 1997;272(6 pt 2):R1972–R1979. [DOI] [PubMed] [Google Scholar]
- 21. Newnham JP, Moss TJ, Padbury JF, et al. The interactive effects of endotoxin with prenatal glucocorticoids on short-term lung function in sheep. Am J Obstet Gynecol. 2001;185(1):190–197. [DOI] [PubMed] [Google Scholar]