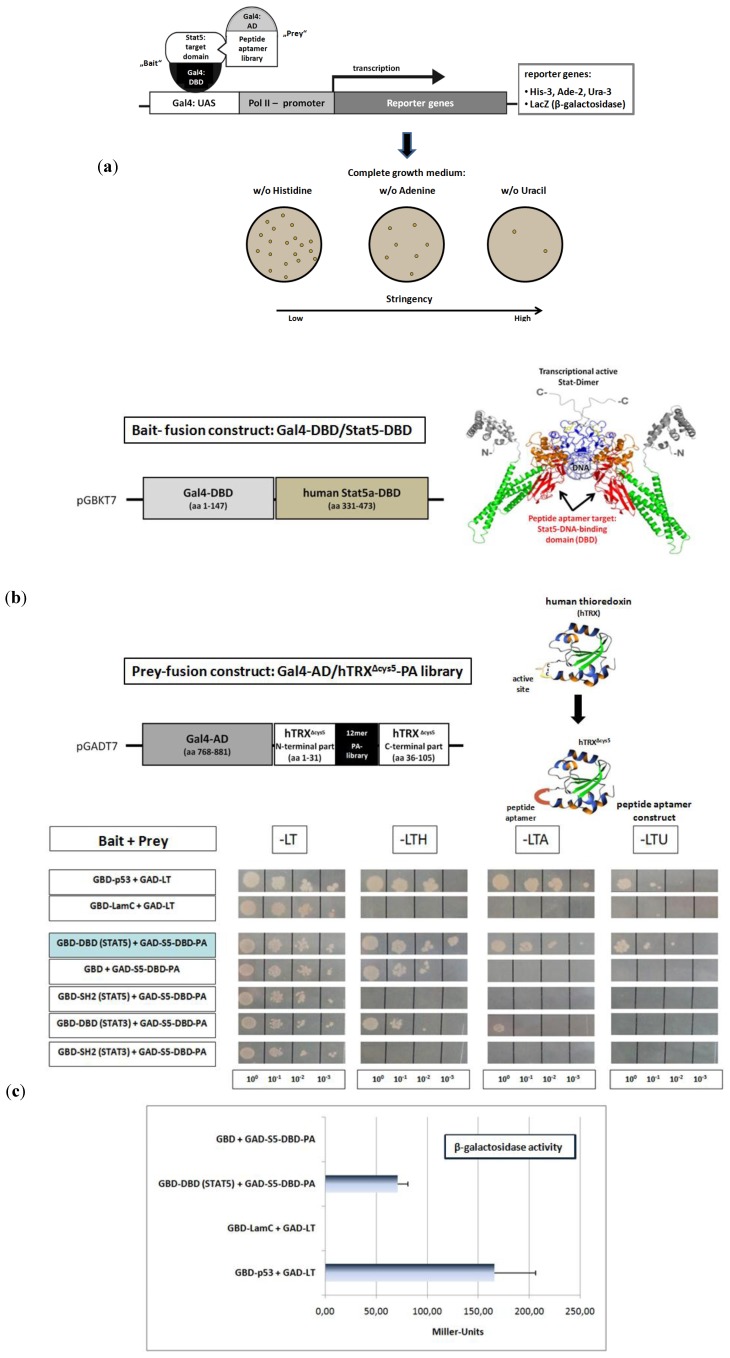
Figure 2.
Identification of a 12mer peptide aptamer sequence (S5-DBD-PA) that specifically binds with high affinity to the DNA-binding domain (DBD) of Stat5. (a) Yeast-two-hybrid (Y2H) screening strategy for the isolation of PA sequences that interact with a functional domain of human Stat5. Y2H screens were performed with a yeast strain (KF1) containing four different Gal4-dependent reporter genes of different stringency. This included genes for the biosynthesis of histidine (His-3), adenine (Ade-2) and uracil (Ura-3) as well as the LacZ-reporter gene for β-galactosidase expression and activity measurement. The corresponding expression vectors (bait and prey) were co-transformed into yeast cells, which were plated and grown under highly stringent conditions on selective media lacking uracil, for identifying the strongest PA interactions. The clone with the best binding properties after revalidation was chosen for further analysis. (b) Schematic representation of the cloned bait and prey fusion proteins used for Y2H screening. The DBD of human Stat5A comprises amino acids 331 to 473, indicated in red in the crystallographic structure of a Stat-dimer bound to DNA [47]. This domain was fused to the Gal4 DNA-binding domain (Gal4-DBD) and used as bait. The complex 12-mer PA library was expressed within the active loop of the modified human thioredoxin scaffold (hTRXΔcys5, schematically indicated by the hTRX crystal structure), which was fused to the Gal4 transcriptional activation domain (Gal4-AD). These random PA sequences presenting fusion proteins were used as prey constructs.(c) The specificity of binding of the identified aptamer (S5-DBD-PA) to the STAT5-DBD was verified after plasmid sequencing and retransformation by plating the yeast cultures in 1:10, 1:100 and 1:1,000 dilutions on selective media lacking leucine (L) and tryptophan (T), which synthesis genes were encoded by the corresponding bait and prey expression vectors, and additionally either histidine (H), adenine (A) or uracil (U). Weak interactions allow growth on -LTH, whereas growth on -LTU requires strong interactions. The interaction of p53 or lamin C with the SV40 Large T antigen served as positive and negative controls. The Gal4-AD (GAD) fused S5-DBD-PA binding properties against the single GAL4-DBD (GBD) and other fused domains of Stat3 and Stat5 (DBD- or SH2-domain) were additionally evaluated. Protein interactions were also quantified by measuring β-galactosidase activity. Results are shown as Miller-Units (n = 3; Ø ± SD).