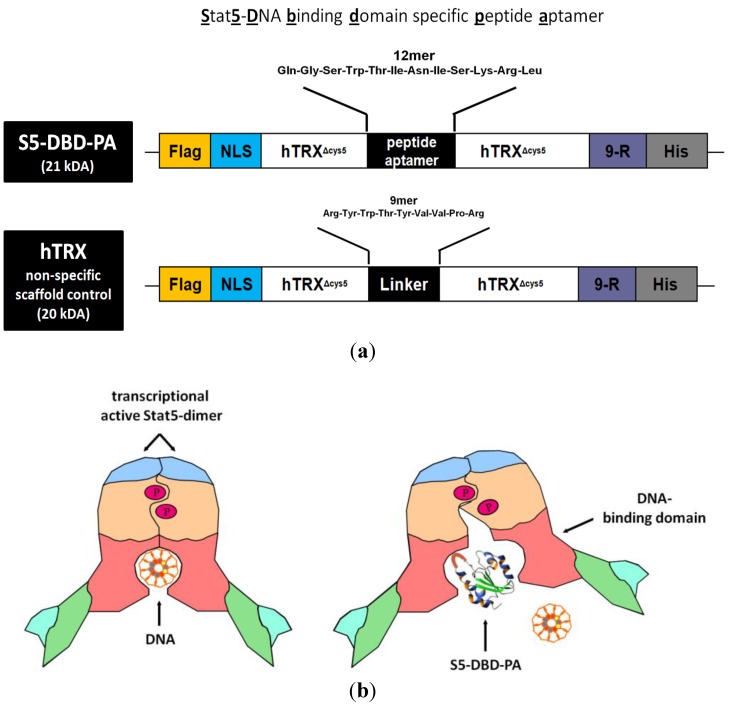
Figure 3.
Domain structure of the S5-DBD-PA protein construct used for viral and protein transduction experiments and a model for its inhibitory function. (a) The Stat5-DBD specific 12mer PA sequence (S5-DBD-PA) was introduced in the protruding active loop of the hTRXΔcys5 protein, which serves as a scaffold for enhanced binding affinity and stability. A protein transduction domain (PTD) consisting of 9 arginines (9-R) is added for intracellular uptake. Additionally a C- terminal histidine tag (His) for purification, a NLS-domain for an enhanced nuclear import and a N-terminal Flag-tag (Flag) for proper detection were added. The complete S5-DBD-PA protein construct has a size of 21 kDa. As a negative control for further analysis the scaffold protein, containing an unspecific 9mer linker sequence instead of the PA sequence was used. This control protein was termed hTRX and has a size of 20 kDa. (b) Model for inhibition of the Stat5-DNA-binding activity by the interacting peptide ligand S5-DBD-PA. The specific interaction of S5-DBD-PA with the Stat5-DBD prevents the binding of Stat5 and its DNA response element. It blocks essential regions for the recognition and binding to GAS DNA.