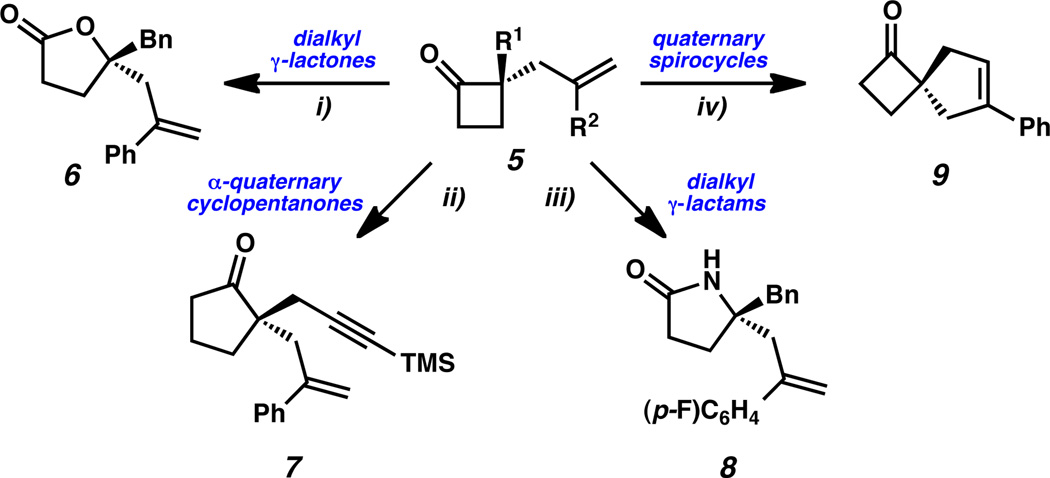
Scheme 2.
Derivation of enantioenriched α-quaternary cyclobutanones. Conditions: i) 5cH2O2 (55 wt% in H2O), 1 M NaOH, MeOH, 23 °C, 80% yield. ii) 5g, TMSCHN2, BF3·Et2O, Et2O, HCl (aq.), DCM, 69% yield, two steps. iii) 5o, HONH2·HCl, pyridine, EtOH; p-TsCl, Et3N, DMAP, DCM, 22% yield, two steps. iv) 5f, Grubbs-Hoveyda G2, PhH, 97% yield.