Table 2.
Catalytic enantioselective cyclobutanone alkylation: (A) scope of α-keto substitutent tolerance; (B) functional diversity incorporated at the 2-allyl position.[a]
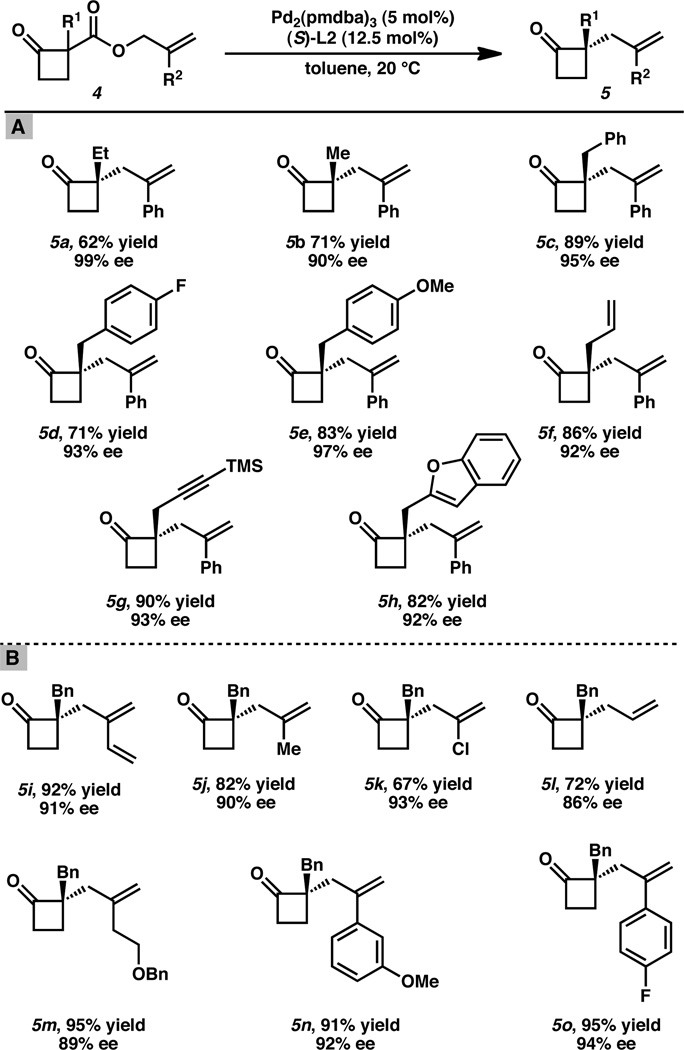 |
Conditions cyclobutanone 4 (1.0 equiv), Pd2(pmdba)3 (5 mol%), (S)-L2 (12.5 mol%) in toluene (0.033 M) at 20 °C for 12–48 h. All reported yields are for isolated products.
