Summary
Background and objectives
Soluble Klotho is an anti-aging phosphaturic protein associated with vascular-renal protection. In vitro and in vivo studies have demonstrated that renin-angiotensin system (RAS) blockade increases soluble Klotho levels. The effect of RAS blockers on soluble Klotho in patients with diabetic kidney disease (DKD) is unknown.
Design, setting, participants, & measurements
Plasma-soluble Klotho was measured in a secondary analysis of a randomized controlled clinical trial performed at a single university hospital center (ClinicalTrials.gov number NCT001715, from March 2003 to September 2006). Seventy-six patients with type 2 diabetes and DKD (all with albuminuria and serum creatinine <1.7 mg/dl) were studied at baseline and at 24 weeks (study end) after randomization to valsartan/hydrochlorothiazide (n=37) or amlodipine (n=39) treatment. Aortic-pulse wave velocity by applanation tonometry and albuminuria (from three timed urine collections) were also measured at baseline and 24 weeks.
Results
Valsartan/hydrochlorothiazide treatment significantly increased mean (± SD) soluble Klotho (from 432.7±179 to 506.4±226.8 pg/ml; P=0.01) and reduced serum phosphate (from 3.25±1.18 to 2.60±0.96 mg/dl; P=0.04) compared with amlodipine (from 430.1±145.8 to 411.9±157.6 pg/ml and from 2.94±0.56 to 2.69±1.52 mg/dl, respectively). There was a significant difference between treatment groups in soluble Klotho (mean 91.9 pg/ml; 95% confidence interval, 19.9 to 162) and serum phosphate levels (mean −0.68 mg/dl; 95% confidence interval, −0.15 to −1.33) with valsartan/hydrochlorothiazide treatment (P=0.03 and P=0.04, respectively). Attained BP was similar in the two groups and levels of soluble Klotho were not associated with aortic-pulse wave velocity and albuminuria, variables that fell significantly only with valsartan/hydrochlorothiazide.
Conclusions
Treatment with a RAS blocker, valsartan, is associated with an increase in soluble Klotho, which may contribute to the BP-independent cardiorenal benefits of these drugs in DKD.
Introduction
Klotho is an aging-suppressor gene encoding a transmembrane protein with an extracellular portion, and is predominantly expressed in the distal renal tubules and in the circulation as a soluble isoform (1,2). This membrane-bound protein works in conjunction with fibroblast growth factor 23 (FGF-23), a bone-derived hormone that regulates urinary phosphate excretion (2–4). The circulating form of Klotho (molecular mass of 130 kD), detectable in plasma and urine, is named soluble or cleaved Klotho.
Soluble (cleaved) Klotho is derived from the proteolytic cleavage of the extracellular portion of the membrane-bound Klotho and consists of two internal repeats, known as KL1 and KL2, respectively. KL1 (molecular mass of 68–70 kD) may be produced through alternative mRNA splicing, but its biologic role is unclear.
In mice, the deletion of the Klotho gene is associated with shortened lifespan, derangement of phosphorus regulation, renal fibrosis, and vascular calcification. There is emerging evidence that soluble Klotho may have biological effects in addition to the transmembrane form because the exogenous replacement of soluble Klotho restores a normal phenotype in Klotho mutant mice (1,2,4). Soluble Klotho confers vascular-renal protection in different experimental models of metabolic and kidney diseases by enhancing antioxidant, antisenescence, and antiapoptotic mechanisms (1,2). Angiotensin II (AngII), a proinflammatory and oxidant agent, is upregulated in a variety of kidney diseases, including diabetic kidney disease. In animal models of kidney disease, AngII decreases renal Klotho expression and this downregulation is prevented by an angiotensin type I receptor blocker (ARB) (5,6).
The effect of an ARB on soluble Klotho, FGF-23, and serum phosphate in humans—specifically, in patients with type II diabetes mellitus, systolic hypertension, and albuminuria—is unknown. Our hypothesis was that an ARB, compared with a calcium channel blocker, would significantly increase soluble Klotho levels in a BP-independent manner in patients with type II diabetes mellitus, systolic hypertension, and albuminuria.
Materials and Methods
Patients with type II diabetes mellitus with systolic hypertension and albuminuria were recruited as previously described to a single-center double-blind randomized controlled study (ClinicalTrials.gov registry number NCT00171561 between March 2003 and September 2006) (7). In brief, patients with type II diabetes mellitus were eligible if they were aged between 40 and 80 years with a recorded history of elevated albumin excretion rate (AER) (albumin/creatinine ratio ≥22 mg/g in men and ≥31 mg/g in women on >3 occasions and/or AER ≥20 μg/min on at least two timed overnight urine collections) and systolic hypertension defined as brachial systolic BP (SBP) ≥140 mmHg and pulse pressure (PP) ≥60 mmHg (a conservative PP criterion for the diagnosis of isolated systolic hypertension) (7). Exclusion criteria included clinical or biochemical evidence of renal impairment (creatinine >1.7 mg/dl), uncontrolled diabetes defined as fasting plasma glucose >200 mg/dl or hemoglobin A1c (HbA1c) >10%, presence of connective tissue diseases known to affect arterial vasculature, atrial fibrillation or other cardiac rhythm disorders, history of nondiabetic or obstructive kidney disease, microscopic or macroscopic hematuria, pregnancy, and history of a cardiovascular or cerebrovascular event in the preceding 12 months.
Patients receiving antihypertensive agents for systolic hypertension were permitted into the trial. BP medications were stopped for a wash-out, run-in phase of 4 weeks, during which all patients received moxonidine (400 μg/d), a central selective imidazoline receptor agonist. Moxonidine has a minimal effect on vessel wall properties and was used to avoid uncontrolled hypertension (SBP ≥180 mmHg) (8). At the end of the run-in period, patients with brachial PP ≥60 mmHg and SBP ≥140 mmHg were randomized to receive 160 mg of valsartan or 5 mg of amlodipine and moxonidine was discontinued. After 4 weeks, a dosage of 25 mg/d of hydrochlorothiazide was added to valsartan and amlodipine was uptitrated to 10 mg. Hydrochlorothiazide was added to 160 mg of valsartan (the maximum dose licensed for use in the United Kingdom) to ensure equivalent BP lowering between the treatment arms. Hydrochlorothiazide has no significant effect on arterial wall properties (9). The use of other antihypertensive agents was not permitted during the trial because they could interfere with study evaluations and interpretation of results.
All measurements and procedures were performed with the patients in the fasted state and having refrained from nicotine, alcohol, and caffeine for at least the previous 10 hours.
Plasma-soluble Klotho (IBL Co. Ltd., Minneapolis, MN) and plasma C-terminal FGF-23 (Immunotopics Inc., San Clemente, CA) were measured in duplicate by ELISA from samples collected at baseline and at the end of the 24 weeks of treatment and were stored at −80°C. Serum phosphate was measured in duplicate by spectrophotometry (Pointe Scientific Inc., Canton, MI). The intra-assay and interassay coefficients of variation for soluble Klotho and FGF-23 ELISA were 2.7% and 6.5% and 4.4% and 6.1%, respectively (10).
The assay we utilized for measuring soluble Klotho has been used in many clinical and experimental studies and in a range of patients and animal models of disease (4,10–13).
The soluble Klotho assay used in our study and other studies to date measures the larger 130 kD cleaved protein. A smaller fragment of 68–70 kD, as a result of alternative mRNA splicing (2), of current unknown significance may be present in the circulation, but is not detected by this assay.
Aortic-pulse wave velocity (Ao-PWV) was determined from carotid and femoral pressure waveforms obtained noninvasively by applanation tonometry (Millar tonometer; Millar Instruments, Houston, TX) using the SphygmoCor system (Atcor, Sydney, Australia) as previously described (7). The within-subject SD of Ao-PWV assessed using this method in our laboratory was 0.5 m/s and the intraobserver and interobserver coefficients of variation were 3.0% and 3.5%, respectively (7). Albumin concentration was measured by immunoturbidimetry using a Cobas Miras Plus analyzer (Roche Diagnostics, Basel, Switzerland) and AER was calculated from the median of three nonconsecutive timed overnight urine specimens collected 1 week before Ao-PWV measurement (7).
Two-sample t tests (paired) were conducted to compare variables in each group at baseline and at the study end, whereas comparison between groups was conducted with an independent sample t test. AER and FGF-23 data were log-transformed before parametric tests. A multivariate linear regression analysis was performed to determine which independent variables were associated with change in soluble Klotho levels (the dependent variable). All statistical analyses were performed using SPSS software (version 17; SPSS Inc., Chicago, IL). All measurements and statistical analyses were performed blinded to treatment groups.
All patients were recruited from the Diabetes Clinic at Guy’s and St Thomas’ Hospitals (London, UK) and provided written informed consent to participate in the study, which was approved by the research ethics committee of Guy’s and St Thomas’ Hospitals and undertaken in adherence to the Declaration of Helsinki.
Results
Of the 103 patients who completed the primary study, 76 patients had samples available for measurement of soluble Klotho. The baseline clinical and biochemical characteristics of the 27 patients who did not have samples available for analyses were not significantly different from the 76 patients studied.
There were no significant baseline differences between the two groups in terms of sex (men comprised 60% of both groups) or ethnic distribution. Selected baseline clinical and biochemical data are shown in Table 1. All patients had serum creatinine <1.41 mg/dl and estimated GFR (eGFR) >45 ml/min per 1.73 m2 calculated by the four-variable Modified Diet in Renal Disease formula. Prior use of antihypertensive drugs was similar in the two groups. The frequency and number of antidiabetic, antiplatelet, and lipid-lowering agents were also similar in the two groups. No patients were taking vitamin D treatment or phosphate binders or calcium or phosphate supplements.
Table 1.
Baseline selected clinical and biochemical data for 76 patients with type II diabetes mellitus, systolic hypertension, and albuminuria randomized to valsartan and hydrochlorothiazide or amlodipine treatment
| Variable | Valsartan/Hydrochlorothiazide (n=37) | Amlodipine (n=39) | Valsartan/Hydrochlorothiazide versus Amlodipine (P Value) |
|---|---|---|---|
| Age (yr) | 60.3±8.5 | 59.3±10.2 | 0.64 |
| Duration of diabetes (yr) | 10.8±7.5 | 10.1±6.7 | 0.76 |
| Body mass index (kg/m2) | 31.4±5.5 | 31.9±5.6 | 0.88 |
| Brachial SBP (mmHg) | 155.5±10.8 | 159.5±10.7 | 0.09 |
| Brachial DBP (mmHg) | 80.3±9.6 | 81.9±9.4 | 0.42 |
| Ao-PWV (m/s) | 12.4±2.4 | 12.2±2.7 | 0.72 |
| HbA1c (%) (mmol/mol) | 7.1 (54) ± 1.0 (11) | 7.6 (60) ± 1.1 (12) | 0.34 |
| Total cholesterol (mg/dl) | 169.9±34.7 | 162.2±38.6 | 0.72 |
| Serum creatinine (mg/dl) | 0.92±0.20 | 0.95±0.22 | 0.64 |
| eGFR (ml/min per 1.73 m2) | 92.3±21.6 | 96.7±23.9 | 0.40 |
| UAER (µg/min) | 31 (18–90) | 25(14–60) | 0.76 |
Data are presented as the mean ± SD or median (interquartile range) unless otherwise stated. SBP, systolic BP; DBP, diastolic BP; Ao-PWV, aortic-pulse wave velocity; HbA1c, hemoglobin A1c; eGFR, estimated GFR; UAER, urine albumin excretion rate.
Table 2 shows the changes in soluble Klotho, serum phosphate, FGF-23, PP, and Ao-PWV from baseline to the study end. Soluble Klotho levels increased significantly only in the valsartan/hydrochlorothiazide group (from 432.7±179 to 506.4±226.8 pg/ml) versus the amlodipine group (from 430.1±145.8 to 411.9±157.6 pg/ml; P=0.04), with a significant mean difference of 91.9 pg/ml between treatment groups (95% confidence interval [95% CI], 19.9 to 162; P=0.03; Figure 1). Concomitantly, phosphate levels fell significantly only in the valsartan/hydrochlorothiazide group (from 3.25±1.18 to 2.60±0.96 mg/dl; P=0.04), with a significant mean difference of −0.68 mg/dl between treatment groups (95% CI, −0.15 to −1.33; P=0.04; Figure 1). In the valsartan/hydrochlorothiazide group, change in soluble Klotho was negatively correlated with change in phosphate (Pearson correlation R2=−0.41; P=0.07) but did not reach the conventional level of statistical significance. FGF-23 levels increased significantly, albeit very modestly, from a median of 17.2 RU/ml (interquartile range [IQR], 11.7–24.9) to 21.9 RU/ml (IQR, 13.0–39.0) with valsartan/hydrochlorothiazide treatment (P=0.01) compared with no significant change observed with amlodipine treatment, which changed from a median of 19.2 RU/ml (IQR, 12.8–23.5) to 18.5 RU/ml (IQR, 13.2–22.8) with no significant difference between treatment groups. Log-transformed FGF-23 data are shown in Table 2.
Table 2.
Selected data from baseline and study end for 76 patients with type II diabetes mellitus, systolic hypertension, and albuminuria randomized to valsartan and hydrochlorothiazide or amlodipine treatment
| Variable | Valsartan/Hydrochlorothiazide (n=37) | Amlodipine (n=39) |
|---|---|---|
| Soluble Klotho (pg/ml) | ||
| Baseline | 432.7±179.0 | 430.1±145.8 |
| Study end | 506.4±226.8 | 411.9±157.6 |
| Change from baseline (95% CI) | 73.7 (19.2 to 128.2) | −18.3 (−29.0 to 65.6)a |
| P value | 0.01 | 0.43 |
| Serum phosphate (mg/dl) | ||
| Baseline | 3.25±1.18 | 2.94±0.56 |
| Study end | 2.60±0.96 | 2.69±1.52 |
| Change from baseline (95% CI) | −0.65 (−0.09 to −1.33) | 0.22 (−0.03 to 0.90)a |
| P value | 0.04 | 0.44 |
| Log FGF-23 (RU/ml) | ||
| Baseline | 1.21±0.18 | 1.31±0.23 |
| Study end | 1.32±0.21 | 1.29±0.24 |
| Change from baseline (95% CI) | 0.11 (0.02 to 0.19) | −0.02 (−0.05 to 0.09) |
| P value | 0.01 | 0.55 |
| Brachial pulse pressure (mmHg) | ||
| Baseline | 76.6±11.5 | 74.1±9.7 |
| Study end | 61.5±13.3 | 61.0±10.2 |
| Change from baseline (95% CI) | −15.1 (−12.4 to −17.8) | −13.1 (−10.0 to −16.2) |
| P value | <0.001 | <0.001 |
| Ao-PWV (m/s) | ||
| Baseline | 12.4±2.4 | 12.2±2.7 |
| Study end | 10.7±2.8 | 11.8±2.8 |
| Change from baseline (95% CI) | −1.7 (−2.3 to −1.1) | −0.5 (−1.3 to 0.04)a |
| P value | <0.001 | 0.22 |
Data are presented as the mean ± SD unless otherwise stated. 95% CI, 95% confidence interval; FGF-23, fibroblast growth factor 23; Ao-PWV-Aortic-pulse wave velocity, FGF-23-Fibroblast growth factor 23.
P<0.05 between treatment groups.
Figure 1.
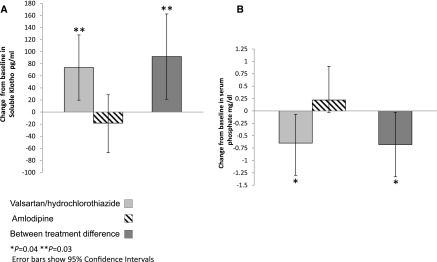
Valsartan/hydrochlorothiazide increases plasma soluble Klotho and reduces serum phosphate. Changes from baseline and between treatment difference in mean soluble Klotho (A) and serum phosphate (B) in 76 patients with type 2 diabetes mellitus, systolic hypertension, and microalbuminuria randomized to valsartan/hydrochlorothiazide (n=37) or amlodipine (n=39) treatment for 24 weeks. Error bars show 95% confidence intervals. *P=0.04; **P=0.03.
Renal function and serum creatinine remained stable during the study, with no significant reduction or differences between the two groups at the study end. There was no significant baseline difference in corrected serum calcium levels between the two groups (9.44±0.40 versus 9.40±0.44 mg/dl; P=0.73) or change in serum calcium levels during the study (data not shown). Changes in soluble Klotho, phosphate, or FGF-23 were not significantly associated with changes in Ao-PWV, BP, or albuminuria.
There were no statistically significant correlations between changes in Klotho levels with Ao-PWV (R2=0.12; P=0.54) or albuminuria (R2=−0.17; P=0.37) observed in the valsartan/hydrochlorothiazide group, in which there was a significant reduction in Ao-PWV and albuminuria. In a multivariate regression analysis model, valsartan/hydrochlorothiazide treatment was the only variable significantly associated with the change in serum Klotho levels (SD change of soluble Klotho: standardized coefficient β=0.29; P=0.04). The following variables were also entered into the multivariate model: age, duration of diabetes, baseline PP, and AER; however, none of these variables were significantly associated with change in soluble Klotho.
Ao-PWV fell significantly from 12.4±2.4 m/s to 10.7±2.8 m/s in the valsartan/hydrochlorothiazide group, with a mean change of −1.7 m/s from baseline (95% CI, −2.3 to −1.1; P<0.001). In contrast, there was no statistically significant reduction observed in the amlodipine group (from 12.2±2.7 to 11.8±2.8 m/s), with a mean change of −0.5 from baseline m/s (95% CI, −1.3 to 0.04; P=0.22). PP fell significantly and similarly by a mean of −15.1 mmHg (95% CI, −17.8 to −12.4) and a mean of −13.1 mmHg (95% CI, −16.2 to −10.0) with valsartan/hydrochlorothiazide and amlodipine, respectively, with no between-group difference.
Albuminuria fell significantly only in the valsartan/hydrochlorothiazide group, which decreased from a median of 31.0 μg/min (IQR, 18.0–90.0) to 17.0 μg/min (IQR, 8.0–42.0) compared with amlodipine, which decreased from a median of 25.0 μg/min (IQR, 14.0–60.0) to 22 μg/min (12.0–70.5) (P=0.03 between groups). Glycemic and lipid control remained stable during the 24 weeks of treatment, with no significant differences between the two groups at the study end (data not shown).
Discussion
Our study is the first to demonstrate that 24-week treatment with an ARB, valsartan, and hydrochlorothiazide significantly increases the plasma levels of soluble Klotho in patients with type II diabetes mellitus with albuminuria and relatively preserved renal function, an effect apparently independent of BP and associated with a reduction in serum phosphate.
Our data, from a secondary analysis, translate in humans the findings of earlier experimental studies demonstrating that angiotensin converting enzyme inhibitors or ARBs increase renal Klotho expression (5,14).
In a recent study conducted in 33 Asian patients with type II diabetes mellitus, 4-week treatment with losartan significantly increased soluble Klotho levels compared with an angiotensin converting enzyme inhibitor, quinapril (15). In the losartan-treated group, the rise in soluble Klotho was paralleled by a reduction in albuminuria (15). However, in this 4-week crossover study, both renin-angiotensin system (RAS) inhibitors were dosed submaximally (50% of maximum dose) and used in combination with other antihypertensive agents in some patients. Furthermore, phosphate, calcium, FGF-23, and Ao-PWV were not measured and albuminuria was determined from a single spot urine sample rather than a timed collection. Surprisingly, the authors reported that, in contrast to other studies, quinapril did not result in a significant reduction in BP or albuminuria (15,16).
Most clinical and laboratory studies in this field demonstrate that raised levels of soluble Klotho (measured by the same assay we utilized) are associated with reduced cardiovascular and renal risk (4). By contrast, renal impairment is characterized by a state of low levels of soluble Klotho (4).
Although previous clinical studies included healthy participants, patients with diabetes, and patients with varying severity of renal impairment (stage 1–5 CKD), the levels of soluble Klotho were not studied in detail in patients with type II diabetes mellitus, albuminuria, and relatively preserved renal function (all had an eGFR >45 ml/min per 1.73 m2 and only 3 of 76 patients had an eGFR <60 ml/min per 1.73 m2) such as in this study. Recent studies in patients with more advanced kidney disease reported conflicting data. Akimoto et al. measured 24-hour urinary Klotho in patients with stage 1–5 CKD (60% had chronic GN as the cause of CKD, and only 16% had CKD caused by diabetic renal disease) and noted that urine Klotho levels and soluble Klotho levels were both significantly higher in stage 1 CKD compared with stage 4 and stage 5 CKD (17). In a multiple regression analysis, eGFR was independently associated only urinary excreted Klotho (17). Shimamura et al. reported higher levels of soluble Klotho in stage 1 CKD compared with stage 2 CKD and stages 3–5 in 292 patients, 10% of whom had diabetic renal disease (12). In contrast, Devaraj et al. demonstrated that nondiabetic participants with renal dysfunction (defined as serum creatinine >2 mg/dl) had higher soluble Klotho levels compared with participants with preserved renal function (18).
There are emerging data that soluble Klotho is an independent risk factor for cardiovascular disease. In a longitudinal study of 804 community-dwelling adults aged >65 years, higher levels of soluble Klotho were independently associated with lower cardiovascular risk and decreased all-cause mortality (13).
Soluble Klotho exerts vascular-renal protective effects independent of the transmembrane form that regulates phosphate excretion in the distal tubules in conjunction with FGF-23 (19). In experimental models of CKD, endogenous stimulation of soluble Klotho or its exogenous replacement can slow down the progression of renal dysfunction, reduce proteinuria, and prevent the vascular calcification induced by high levels of phosphate (2,4). Importantly, soluble Klotho may induce phosphaturia by FGF-23–independent mechanisms (2,4).
In patients with albuminuria, high phosphate levels, even within the normal range, are known to promote progression of renal disease and to attenuate the renoprotective effect of RAS inhibitors (20). Increasing evidence from in vitro, clinical, and epidemiologic studies indicates that elevated serum phosphorus levels per se are associated with vascular calcification and cardiovascular mortality (21).
We observed a significant reduction in serum phosphate with RAS blockade in our study, which negatively correlated with changes in soluble Klotho. This correlation (R2=0.41; P=0.07), however, did not reach the conventional level of statistical significance. Because soluble Klotho has a known phosphaturic action, our results provide support to the assay we used, which is important because some uncertainty currently exists regarding the validity of soluble Klotho assays.
The mechanisms explaining our results need to be further investigated. Our results may be explained by increased renal expression of Klotho and its cleavage process; however, this needs to be confirmed in further studies. The assay we used detects soluble (cleaved) Klotho (130 kD) in the circulation. A smaller fragment of 68–70 kD, of unknown significance, is also present in the circulation but is not detected by the assay we utilized.
It is established that increased oxidative stress reduces the expression of Klotho and we speculate that a reduction in oxidative stress with valsartan may help explain, at least in part, our results because RAS blockade is known to reduce reactive oxygen species and inhibit pathways that promote oxidative stress (22). Because we did not directly measure changes in oxidative stress, further studies are needed to confirm our hypothesis/speculation.
We added a thiazide diuretic, hydrochlorothiazide, to ensure equivalent BP control in both study arms of our trial. Although there is no current evidence that thiazide diuretics affect renal Klotho expression, the possibility of an effect of hydrochlorothiazide on this protein, which is expressed in the distal renal tubules, cannot be excluded because we did not measure the levels of Klotho before hydrochlorothiazide was added to valsartan.
Thiazide diuretics are known to increase serum calcium levels and reduce urinary calcium excretion (23). We did not measure urinary calcium excretion in our study so an effect of hydrochlorothiazide on this measure cannot be excluded; however, we did not note any significant change in serum calcium levels with valsartan/hydrochlorothiazide treatment.
Thiazide diuretics do not demonstrate the BP-independent pleiotropic benefits observed with RAS blockers (9,24,25). Moreover, the salt and water depletion that would ensue from thiazide treatment activate the RAS and the autonomic nervous system (26). Therefore, the concomitant use of an antihypertensive agent like hydrochlorothiazide would, if anything, result in an underestimation of the effects of valsartan treatment on Klotho.
We did not measure vitamin D, a negative regulator of the RAS (27). Interestingly, recent data suggest that vitamin D may have cardiorenal protective effects by restoring Klotho levels (28). We also did not measure parathyroid hormone (PTH) levels or fractional excretion of phosphate (FEPi) in our patients. There are limited data on the effects of RAS blockade on PTH and FEPi. In healthy participants, infusion of AngII increases PTH secretion; however, no change in FEPi was reported in this study (29). In 16 children and young adults with T1DM and microalbuminuria, enalapril treatment decreased FEPi; however, to our knowledge, there are no data available in patients with type II diabetes mellitus such as those we studied (30). The effects, if any, of calcium channel blockers on FEPi or PTH have also not been studied in patients with type II diabetes mellitus.
We did measure levels of FGF-23, another important phosphaturic hormone, but we did not observe a significant difference in FGF-23 levels between treatment groups or a correlation between FGF-23 and phosphate or Klotho levels.
In our cohort of patients with type II diabetes mellitus and relatively preserved renal function, we did not observe any significant associations between soluble Klotho and changes in BP, arterial stiffness, and albuminuria. However, our study was not designed to test these associations and our sample size may not have been large enough. In an observational study of 114 Japanese patients with CKD where >50% of participants had an eGFR <45 ml/min per 1.73 m2, the authors noted a significant association between low Klotho levels and raised arterial stiffness and markers of atherosclerosis (11). However, only 11% of participants had diabetes and the authors used ankle-brachial pulse wave velocity to measure arterial stiffness, rather than Ao-PWV, which is the recognized gold standard technique (31).
In patients with type II diabetes mellitus and albuminuria, RAS blockade results in cardiorenal protection independently of BP changes (32). In our study, the RAS blocker valsartan significantly increases soluble Klotho levels and reduces serum phosphate, effects that appear to be independent of its BP-lowering actions.
These results, which need to be confirmed in larger studies, suggest that elevations in soluble Klotho levels may at least in part contribute to the BP-independent benefits observed with RAS blockers in patients with type II diabetes mellitus and albuminuria.
Disclosures
None.
Acknowledgments
We thank the study participants and research nurses, without whom this work would not have been possible.
Some of this work was supported by a grant from Novartis Pharma AG (Basel, Switzerland).
Footnotes
J.K. and G.M. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Maltese G, Karalliedde J: The putative role of the antiageing protein klotho in cardiovascular and renal disease. Int J Hypertens 2012: 757469, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuro-o M: Klotho in health and disease. Curr Opin Nephrol Hypertens 21: 362–368, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Bernheim J, Benchetrit S: The potential roles of FGF23 and Klotho in the prognosis of renal and cardiovascular diseases. Nephrol Dial Transplant 26: 2433–2438, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Hu MC, Shiizaki K, Kuro-o M, Moe OW: Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, Ohashi N, Kobori H, Kuro-o M, Yang CW: Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplan 26: 800–813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Karalliedde J, Smith A, DeAngelis L, Mirenda V, Kandra A, Botha J, Ferber P, Viberti G: Valsartan improves arterial stiffness in type 2 diabetes independently of blood pressure lowering. Hypertension 51: 1617–1623, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Törnig J, Amann K, Ritz E, Nichols C, Zeier M, Mall G: Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure: The effects of ramipril, nifedipine and moxonidine. J Am Soc Nephrol 7: 667–675, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Asmar RG, Benetos A, Chaouche-Teyara K, Raveau-Landon CM, Safar ME: Comparison of effects of felodipine versus hydrochlorothiazide on arterial diameter and pulse-wave velocity in essential hypertension. Am J Cardiol 72: 794–798, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y: Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun 398: 513–518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H, Makino H: A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE 8: e56695, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16: 722–729, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L: Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci 66: 794–800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Q, Lin S, Tang R, Veeraragoo P, Peng W, Wu R: Role of fosinopril and valsartan on Klotho gene expression induced by angiotensin II in rat renal tubular epithelial cells. Kidney Blood Press Res 33: 186–192, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Lim SC, Liu JJ, Subramaniam T, Sum CF: Elevated circulating alpha-klotho by angiotensin II receptor blocker losartan is associated with reduction of albuminuria in type 2 diabetic patients [published online ahead of print February 4, 2013]. J Renin Angiotensin Aldosterone Syst10.1177/1470320313475905 [DOI] [PubMed] [Google Scholar]
- 16.Dominguez LJ, Barbagallo M, Kattah W, Garcia D, Sowers JR: Quinapril reduces microalbuminuria in essential hypertensive and in diabetic hypertensive subjects. Am J Hypertens 8: 808–814, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, Iwazu K, Komada T, Otani N, Morishita Y, Ito C, Shiizaki K, Ando Y, Muto S, Kuro-o M, Kusano E: Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol 13: 155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devaraj S, Syed B, Chien A, Jialal I: Validation of an immunoassay for soluble Klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol 137: 479–485, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Chen TH, Kuro-O M, Chen CH, Sue YM, Chen YC, Wu HH, Cheng CY: The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur J Pharmacol 698: 67–73, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Zoccali C, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Mallamaci F, Remuzzi G, REIN Study Group : Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 22: 1923–1930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shroff R: Phosphate is a vascular toxin. Pediatr Nephrol 28: 583–593, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Leiter LA, Lewanczuk RZ: Of the renin-angiotensin system and reactive oxygen species type 2 diabetes and angiotensin II inhibition. Am J Hypertens 18: 121–128, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Middler S, Pak CY, Murad F, Bartter FC: Thiazide diuretics and calcium metabolism. Metabolism 22: 139–146, 1973 [DOI] [PubMed] [Google Scholar]
- 24.Kool MJ, Lustermans FA, Breed JG, Struyker Boudier HA, Hoeks AP, Reneman RS, Van Bortel LM: The influence of perindopril and the diuretic combination amiloride+hydrochlorothiazide on the vessel wall properties of large arteries in hypertensive patients. J Hypertens 13: 839–848, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Zhou MS, Schulman IH, Jaimes EA, Raij L: Thiazide diuretics, endothelial function, and vascular oxidative stress. J Hypertens 26: 494–500, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Safar ME, London GM: The arterial system in human hypertension. In: Textbook of Hypertension, edited by Swales JS, London, Blackwell Scientific Publications, 1994, pp 85–102 [Google Scholar]
- 27.de Borst MH, Vervloet MG, ter Wee PM, Navis G: Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 22: 1603–1609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM: Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int 82: 1261–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant FD, Mandel SJ, Brown EM, Williams GH, Seely EW: Interrelationships between the renin-angiotensin-aldosterone and calcium homeostatic systems. J Clin Endocrinol Metab 75: 988–992, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Yüksel H, Darcan S, Kabasakal C, Cura A, Mir S, Mavi E: Effect of enalapril on proteinuria, phosphaturia, and calciuria in insulin-dependent diabetes. Pediatr Nephrol 12: 648–650, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, European Network for Non-invasive Investigation of Large Arteries : Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Karalliedde J, Viberti G: Evidence for renoprotection by blockade of the renin-angiotensin-aldosterone system in hypertension and diabetes. J Hum Hypertens 20: 239–253, 2006 [DOI] [PubMed] [Google Scholar]


