Figure 2. Characterization of the endogenously expressed Bmpr2 mutant product in pulmonary endothelial cells from Bmpr2 ΔEx2/+ mice.
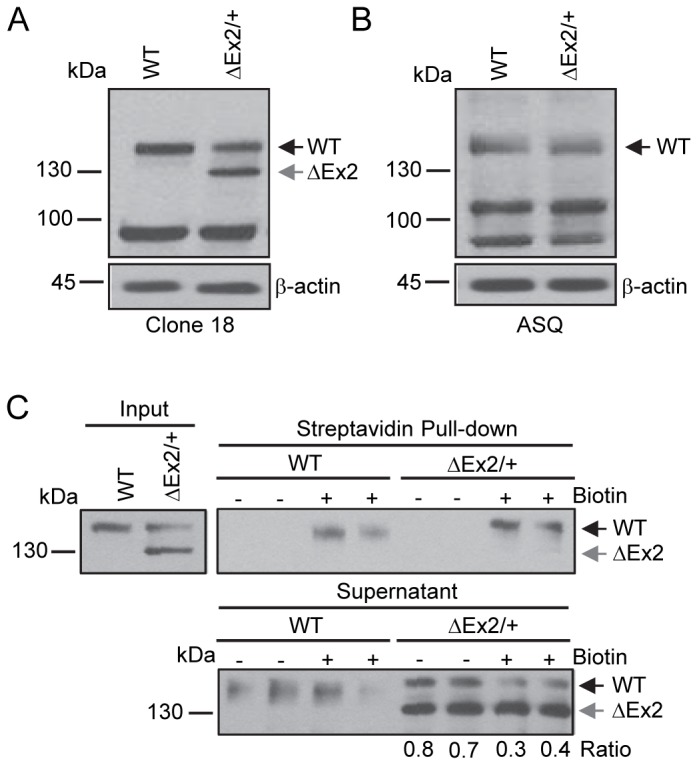
Studies were performed using conditionally immortalized PECs (ciPECs) isolated from wild type control and Bmpr2 ΔEx2/+ mice and replicated at least three times. A, Western blot using the Clone 18 anti-BMPR2 antibody in wild type (WT) and Bmpr2 ΔEx2/+ (ΔEx2/+) ciPEC lysates. Both cell lines expressed a 150 kDa wild type Bmpr2 product (WT). Bmpr2 ΔEx2/+ ciPECs also expressed a 130 kDa product (ΔEx2). B, Western blot using ASQ anti-BMPR2 antibody. Wild type control and Bmpr2 ΔEx2/+ ciPECs expressed 150 kDa WT Bmpr2, but the 130 kDa product was not detected. C, Cell surface expression of Bmpr2 in ciPECs. ciPECs were labeled with membrane impermeable biotin and cell surface expression of Bmpr2 detected in streptavidin pull-down of cell lysates. Anti-BMPR2 Clone 18 antibody detected the 150 kDa wild type Bmpr2 in control and Bmpr2 ΔEx2/+ ciPECs after streptavidin pull-down but not the 130kDa Bmpr2ΔEx2 mutant product. Lower panel, Western blot for Bmpr2 in the supernatant remaining after depletion of cell surface proteins by streptavidin pull-down. The ratios of 150 kDa WT Bmpr2 and the 130 kDa Bmpr2ΔEx2 mutant band intensities in Bmpr2 ΔEx2/+ ciPECs supernatants after depletion of cell surface proteins are indicated below the lower panel.
