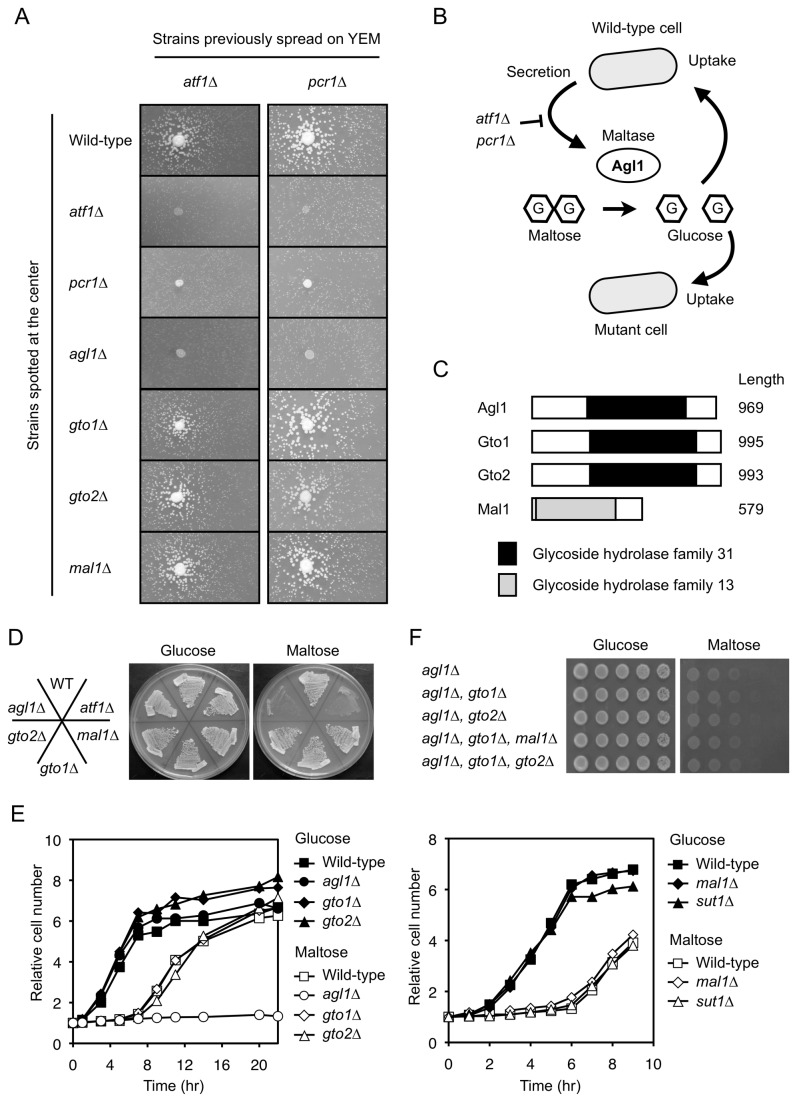
Figure 2. Agl1 is responsible for efficient maltose utilization.
(A) Suppression of the slow growth of atf1∆ and pcr1∆ mutants on maltose-containing medium by neighboring colonies requires the agl1 gene. Strains (indicated on the left) were spotted onto the centers of YEM plates on which atf1∆ or pcr1∆ cells had been spread, and were incubated for 3 days at 30°C. (B) Model describing how neighboring wild-type colonies suppress the slow growth of mutant cells. Wild-type cells secrete the extracellular maltase Agl1 in an Atf1- and Pcr1-dependent manner. Agl1 hydrolyzes maltose into glucose, which is then taken up and utilized by the neighboring mutant cells. (C) Schematic representation of the four possible maltases in fission yeast. The positions of the glycoside hydrolase domains and the lengths (number of amino acids) of each protein are indicated. (D) The agl1∆ mutant grows slowly on maltose-containing plates. The indicated strains were streaked onto plates containing the indicated sugar and incubated at 30°C for 3 days. (E) Growth of the agl1∆ mutant does not recover following switching from glucose- to maltose- containing medium. Cells of the indicated strains exponentially growing in YE were washed twice, resuspended in YE or YEM, and incubated at 30°C for the indicated number of hours. (F) Additional deletion of possible maltase-encoding genes does not enhance the slow growth phenotype of agl1∆ cells on YEM plates. The indicated strains were serially diluted, spotted onto plates containing the indicated sugars, and incubated at 30°C for 5 days. Note: wild-type cells must not be spotted onto the same plates in this experiment because they secrete Agl1 and thereby affect the growth of neighboring mutant colonies.