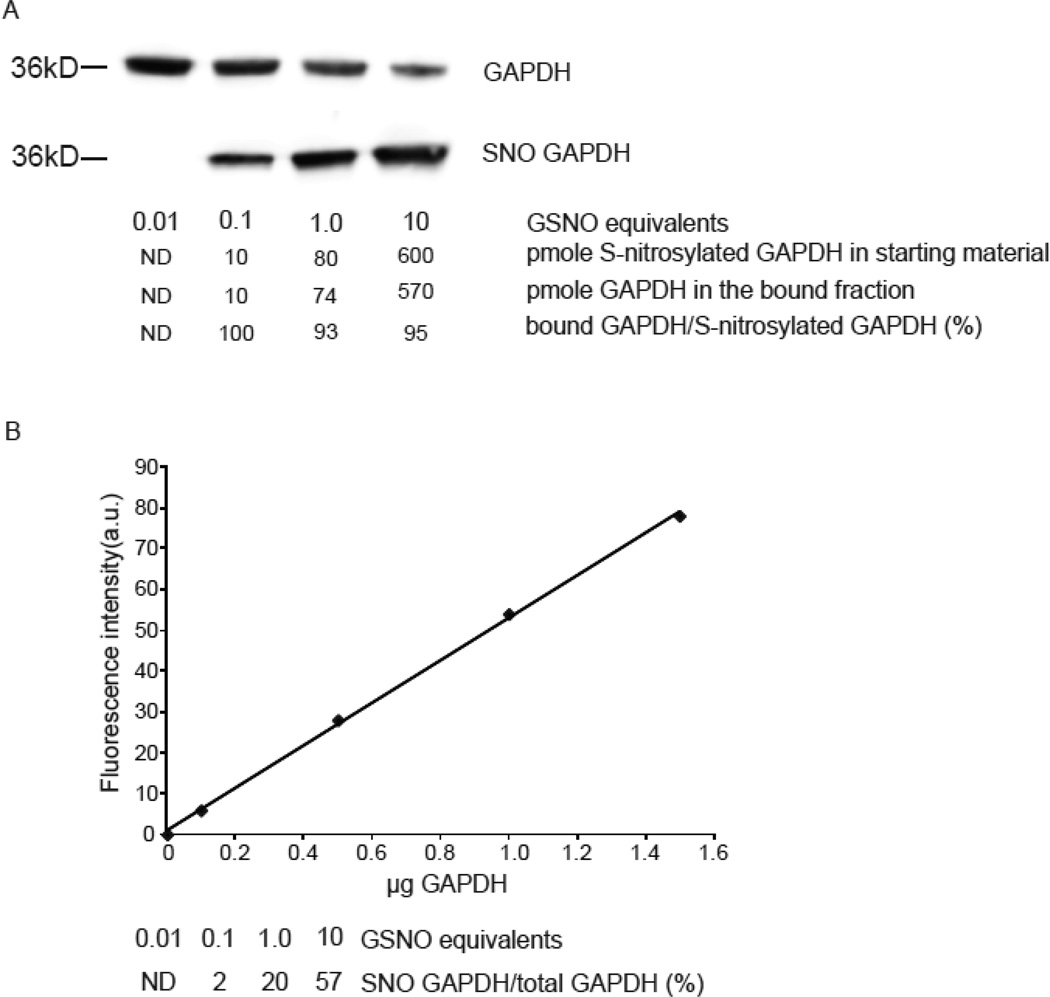
Figure 3. Phenylmercury resin enriches quantitatively for S-nitrosocysteine.
(A) Representative western blot with anti-GAPDH antibodies in bound and unbound fractions collected after phenylmercury resin-assisted capture. GAPDH (140 µM) was exposed to the indicated equivalents of GSNO and 50 µg from each preparation was used for phenylmercury-assisted capture. To avoid signal saturation, 1 µg from the unbound fraction was loaded to the gels. For the same reason, the bound fractions were loaded as follows; 100%, 100%, 20% and 10% of the volume of bound fraction corresponded to GAPDH treated with 0.01, 0.1, 1 and 10 equivalents of GSNO respectively. The levels of S-nitrosocysteine per µg of protein were 0.2, 1.6 and 12 pmole as quantified by reduction of S-NO bond followed by chemiluminescence-based detection of liberated NO equivalents. (B) GAPDH was used to titrate antibody binding and construct this standard curve. Densitometric analysis was employed to determine the fraction of GAPDH present in the bound and unbound fractions. The experiment repeated once more with similar results.