Table 10.
Oxidation of alcohols with copper catalysts using oxygen.
| Entry | Substrates | Co-oxidant | Ligand/Complex | Additive | Reaction conditions | Yields | # of examples | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | Benzylic | 1 mol% CuCl2 | 1.2 equiv CsCO3 | Toluene, 40 °C, O2, 12 h | 72–94% | 9 | 431 | |
| 2 | Benzylic 1° Aliphatic |
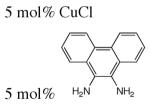
|
0.25 M NaOH | H2O, 100 °C, 10 atm O2, 3 h | 57–99% conv | 5 | 432 | |
| 3 | Propargylic | 10 mol% Cu-nanoparticles 10 mol% bipyridine |
PhMe, air, 80 °C, 8 h | 80–95 | 6 | 433 | ||
| 4 | Benzylic Allylic 1° Aliphatic 2° Aliphatic | Graphite electrode, −0.55 V | 0.03 mol% 2,2'-bisquinoline polymer | 0.15 M LiClO4, 0.05 M Bu4NBF4 | CH2Cl2, air, rt | 85–94% | 5 | 434 |
| 5 | Benzylic | 2 equiv CuCl 2 equiv phen |
2 equiv K2CO3 | Benzene, reflux, O2 | 83–93% | 3 | 435 | |
| 6 | Benzylic | 2 equiv CuCl 2 equiv phen |
2 equiv K2CO3 | Benzene, reflux, O2 | 65–86% | 4 | 436 | |
| 7 | Benzylic | 5 mol% Cu(OAc)2 5 mol% phen |
1:1 DMF/Benz ene, reflux, O2, 2 h | 54–70% | 3 | 437 | ||
| 8 | Benzylic |
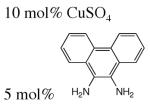
|
NaOH (pH 13.2) | H2O, 80 °C, 10 bar O2, | 54–74% | 5 | 438 |
