Table 5.
Glaser Hay dimerization reactions.
| entry | substrate | conditions | yield(%) | ref |
|---|---|---|---|---|
| 1 |
|
8 mol% Cu(OAc)2·H2O, TMEDA, acetone, air, rt, 2h | 91 | 145 |
| 2 |
|
CuCl, TMEDA, O2, MeOH, THF | 81a | 130 |
| 3 |
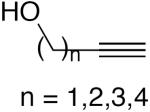
|
5 mol% CuI, TMEDA, DME, O2, 55 °C, overnight | ≥90 | 146 |
| 4 |
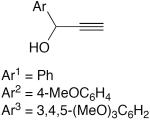
|
15 mol% CuCl, 30 mol% pyridine, MeOH, O2, rt | Ar1 = 91 | 147 |
| Ar2 = 93 | ||||
| Ar3 = 75 | ||||
| 5 |
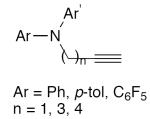
|
10 mol% CuCl, TMEDA, MeOH, air, rt, 24 h | 60–72 | 148 |
| 6 |
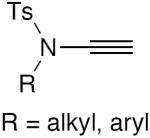
|
10 mol% CuI, TMEDA, O2, acetone, rt, 3 h | 84–100 | 149 |
| 7 |
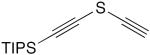
|
CuCl, TMEDA, air, CHCl3 | 94 | 150 |
| 8 |
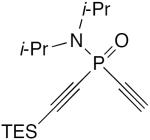
|
10 mol% CuI, 20 mol% TMEDA, acetone, air, rt | 67 | 151 |
| 9 |
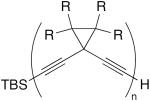
|
3 equiv CuCl, TMEDA, acetone, O2, 1 h, rt | 76 (n = 3, R = Me) | 152 |
| 86 (n = 3, R = H) | ||||
| 95 (n = 6, R = H) | ||||
| 10 |
|
4 mol% CuCl, TMEDA, acetone, O2, rt, 6 h | 84 | 153 |
| 11 |
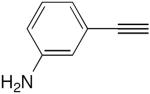
|
14 mol% CuCl, TMEDA, acetone, O2, rt, 4 h | 52 | 154 |
| 12 |
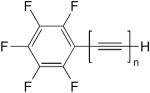
|
CuCl, TMEDA, CH2Cl2, O2 | 50a (n = 2) | 155 |
| 44a (n = 3) | ||||
| 19a (n = 4) | ||||
| 13 |
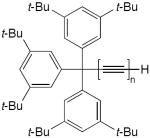
|
CuCl, TMEDA, CH2Cl2, O2 | 92 (n = 2) | 156 |
| 66 (n = 3) | ||||
| 97 (n = 4) | ||||
| 14 |
|
40 mol% CuCl, pyridine, air, 24 h, rt | 95 | 157 |
| 15 |
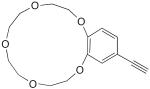
|
25 mol% CuCl, pyr, air, 40 °C, 1.5 h | 60 | 158 |
| 16 |
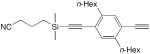
|
1 equiv CuCl, TMEDA, CH2Cl2, air, 30 °C, 2–4 h | 99 | 159 |
| 17 |
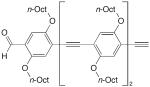
|
6.3 equiv CuCl, TMEDA, CH2Cl2, air, rt, 12 h | 91 | 160 |
| 18 |
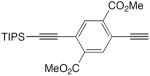
|
CuCl, TMEDA, CH2Cl2, air | 68 | 161 |
| 19 |
|
CuCl, TMEDA, air, acetone, rt, overnight | 43 | 162 |
| 20 |
|
12 mol% CuCl, TMEDA, chlorobenzene, O2, rt, 3d | 50 | 163 |
| 21 |
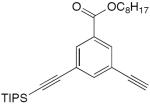
|
4.8 equiv CuCl, TMEDA, acetone, O2, rt, 48 h | 88 | 164 |
| 22 |
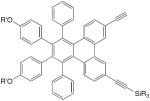
|
CuCl, TMEDA, CH2Cl2, O2, rt, 24 h | 91 | 165 |
| 23 |
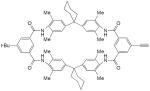
|
30 mol% CuCl, O2, DMF, rt, 12 h | 26 | 166 |
| 24 |
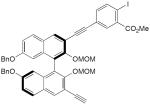
|
CuCl, TMEDA, O2, CH2Cl2, rt | 99 | 167 |
| 25 |
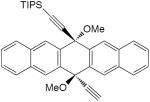
|
1 equiv CuCl, TMEDA, CH2Cl2, O2, rt, 4 h | 60 | 168 |
| 26 |
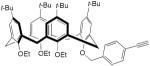
|
5 mol% CuCl, TMEDA, toluene, 65 °C, 1.5 h | 45 | 169 |
| 27 |
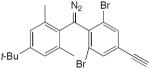
|
catalytic CuCl(OH)·TMEDA, CH2Cl2, O2, rt, overnight | 57 | 170 |
| 28 |
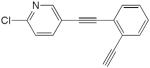
|
20 mol% CuCl, pyr, O2, rt, 24 h | 86 | 171 |
| 29 |
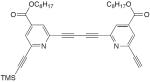
|
30 mol% CuCl, TMEDA, O2, acetone, rt, 48 h | 89 | 172 |
| 30a |
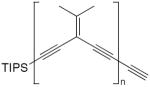
|
4 equiv CuI, TMEDA, CH2Cl2, O2, rt | 96a (n= 1) | 173 |
| 78a (n = 2) | ||||
| 68a (n = 3) | ||||
| 31 |
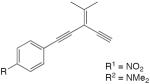
|
CuCl, TMEDA, CH2Cl2, rt, O2 | R1 = 87 | 174 |
| R2 = 68 | ||||
| 32 |
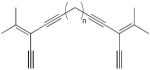
|
4 equiv CuCl, TMEDA, CH2Cl2, O2, rt | 32a (n =2) | 175 |
| 20a (n = 3) | ||||
| 34a (n = 4) | ||||
| 34a (n = 5) | ||||
| 22a (n = 7) | ||||
| (cyclic dimers) | ||||
| 33 |
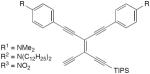
|
CuCl, TMEDA, O2, CH2Cl2 | R1 = 70 | 176 |
| R2 = 86 | ||||
| R3 = 54 | ||||
| 34 |
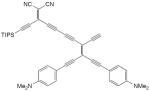
|
CuCl, TMEDA, air, CH2Cl2, rt, 6 h | 70 | 177 |
| 35 |
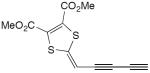
|
5 equiv CuCl, TMEDA, O2, CH2Cl2 | 64a | 178 |
| 36 |
|
CuCl, TMEDA, O2, CH2Cl2 | R1 = 65a | 132 |
| R2 = 55a | ||||
| R3 = 69a | ||||
| 37 |
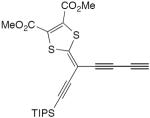
|
CuCl, TMEDA, air, CH2Cl2, rt | 51a | 132 |
| 38 |
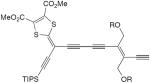
|
CuCl, TMEDA, air, CH2Cl2 | 48a | 132 |
| 38 |
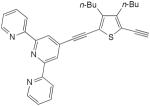
|
10 equiv CuCl, 5 equiv CuCl2, O2, DMF, rt, 4d | 82 | 179 |
| 39 |
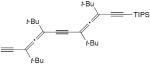
|
CuCl, TMEDA, air, DCE, 50 °C | 91 | 180 |
| 40 |
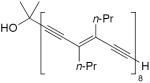
|
2.8 equiv CuCl, TMEDA, CHCl3, O2, rt | 68 | 181 |
| 41 |
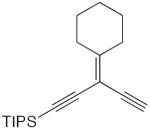
|
50 mol% CuCl, TMEDA, air, CH2Cl2, rt | 86 | 182 |
| 42 |
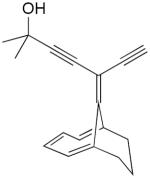
|
CuCl, TMEDA, acetone, air, 17 h, rt | 93 | 183 |
| 43 |
|
2.6 equiv CuCl, TMEDA, CH2Cl2, air, overnight | 93 | 184 |
| 44 |
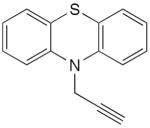
|
10 mol% CuCl, TMEDA, O2, isopropanol, acetone, rt, 5 h | 81 | 185 |
| 45 |
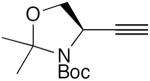
|
20 mol% CuCl, 20 mol% TMEDA, acetone, O2, 35 °C, 1.5 h | 82 | 186 |
| 46 |
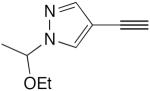
|
14 mol% CuCl, pyr, air, 40 °C, 6.5 h | 36 | 187 |
| 47 |
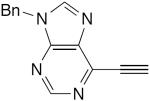
|
20 mol% CuCl, TMEDA, DME, rt, air, overnight | 59 | 188 |
| 48 |
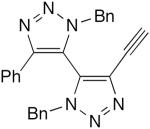
|
CuCl, TMEDA, O2, CH2Cl2, rt | 81 | 189 |
| 49 |
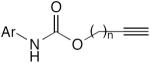
|
10 mol% CuI, pyrrolidine, air, rt | 48–90 | 190 |
| 50 |
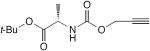
|
1.1 equiv CuCl, air, TMEDA, acetone, rt, 5 h | 64 | 191 |
| 51 |
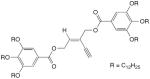
|
3.6 equiv CuCl, TMEDA, CH2Cl2, O2, rt, overnight | 83 | 192 |
| 52 |
|
2.5 equiv CuCl, TMEDA, CH2Cl2, air, rt, 15 min | yield not reported | 193 |
| 53 |
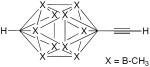
|
0.5 mol% CuCl, DBU, pyridine, O2, 3 h, 45 °C | 96 | 194 |
| 54 |
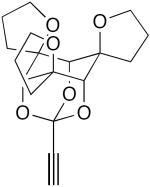
|
1 equiv CuCl, TMEDA, CH2Cl2, air, 4 h, rt | 100 | 195 |
| 55 |
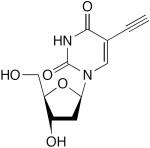
|
2 equiv CuCl, TMEDA, O2, H2O, rt 3 d | 68 | 196 |
| 56 |
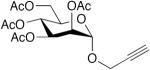
|
CuCl, TMEDA, DMF, O2, 40 °C, 4 h | 99 | 197 |
| 57 |
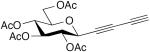
|
CuCl, TMEDA, acetone, O2, rt, 4h | 96 | 198 |
| 58 |
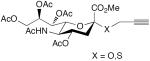
|
30 mol% CuCl, TMEDA, DMF, O2, 40 °C, 2 h | 86 (X = O) | 199 |
| 83 (X = S) | ||||
| 59 |

|
42 mol% CuCl, TMEDA, acetone, O2, rt, | 25 | 200 |
| 60 |
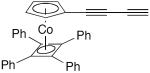
|
50 mol% CuCl, TMEDA, acetone, O2, 4 h, rt | 95 | 201 |
| 61 |
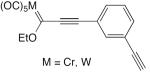
|
superstoich. CuCl, TMEDA, acetone, air, rt, 1.5 h | 60 (R = Cr) | 202 |
| 55 (R = W) | ||||
| 62 |
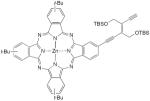
|
1 equiv CuCl, TMEDA, CH2Cl2, O2, 4Å mol sieves, 12 h, rt | 75 | 203 |
| 63 |
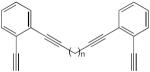
|
1.05 equiv CuCl, DBU, pyridine O2, 45 °C, 2h | >72 (n = 2) | 204 |
| >87 (n = 4) | ||||
| (cyclic dimer) | ||||
| 64 |
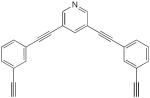
|
11 mol% CuCl, pyridine, O2, rt, 30 h, 32% | 32 (cyclic dimer) | 205 |
| 65b |
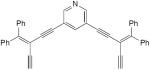
|
7.5 equiv CuI, TMEDA, CH2Cl2, air, rt | 51 (cyclic dimer) | 206 |
| 66 |
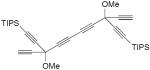
|
CuCl, TMEDA, air, CH2Cl2, rt, 18 h | 72 (mixture of four diastereomer ic cyclic dimers) | 207 |
| 67 |
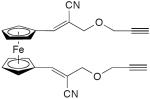
|
2 equiv CuCl2, TMEDA, acetone, O2, rt, 5 h | 32 (cyclic dimer) | 208 |
| 68 |
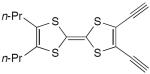
|
CuCl, TMEDA, CH2Cl2, air, 0 °C | 71a (cyclic trimer) | 209 |
Substrate coupled directly after deprotection from silyl-protected precursor.
For cyclodimerization of a similar substrate with excess copper catalyst see ref 210.
