Figure 3. Dihydrogen transfer between arynes and cyclic hydrocarbons.
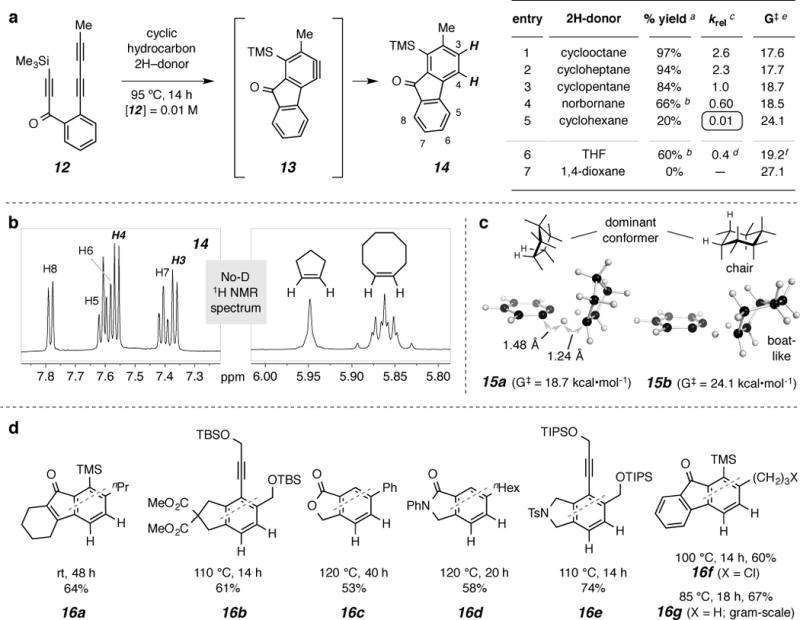
a. Relative efficiency (% yield and krel vs. cyclopentane) of various hydrocarbon (and cyclic ether) 2H-donors for the reduction of aryne13 to arene14. For tabular inset notes a-f see Supplementary Information. b, A representative No-D 1H NMR22 spectrum [this of the reaction solution arising from heating 12 (at 10 mM) in a 1.5:1 molar ratio of cyclopentane:cyclooctane at 95 °C]; thisshows the overall efficiency of the reaction and validates the krel value (1:2.6) obtained as described above. c, Computed TS geometries and ΔG‡ for transfer of two hydrogen atoms to benzyne (3) from cyclopentane (15a) and cyclohexane (15b). d, Reduced benzenoid products 16a-g generated by heating the triyne precursor in cyclooctane under the indicated conditions (starting substrate concentration of 10mM).
