Abstract
The enzyme linked immunospot (ELISpot) assay is a fundamental tool in cellular immunology, providing both quantitative and qualitative information on cellular cytokine responses to defined antigens. It enables the comprehensive screening of patient derived peripheral blood mononuclear cells to reveal the antigenic restriction of T-cell responses and is an emerging technique in clinical laboratory investigation of certain infectious diseases. As with all cellular-based assays, the final results of the assay are dependent on a number of technical variables that may impact precision if not highly standardised between operators. When studies that are large scale or using multiple antigens are set up manually, these assays may be labour intensive, have many manual handling steps, are subject to data and sample integrity failure and may show large inter-operator variability. Here we describe the successful automated performance of the interferon (IFN)-γ ELISpot assay from cell counting through to electronic capture of cytokine quantitation and present the results of a comparison between automated and manual performance of the ELISpot assay. The mean number of spot forming units enumerated by both methods for limiting dilutions of CMV, EBV and influenza (CEF)-derived peptides in six healthy individuals were highly correlated (r >0.83, p<0.05). The precision results from the automated system compared favourably with the manual ELISpot and further ensured electronic tracking, increased throughput and reduced turnaround time.
Keywords: ELISpot, CD8+ T-cells, IFN-γ, Automation, High-throughput, HIV
1. Introduction
The enzyme linked immunospot (ELISpot) assay was introduced in 1983 to detect cellular immunoglobulin production and then adapted to quantitate cytokines and other effector molecules released by individual cells in response to antigenic stimulation ex-vivo. The ELISpot is most commonly used for the detection and functional characterisation of antigen-specific immune responses in the fields of infectious disease, vaccinology and tumour immunology (Cox et al., 2006). The sensitivity of the assay enables the direct enumeration of low-level cytokine-secreting cells, a property which has been utilized in clinical laboratory practice in the novel tuberculosis T-SPOT assay (Lalvani, 2007). Further, the capacity for functional assessment of multiple samples in one assay makes the ELISpot ideal for large scale studies and high-throughput analysis (Kreher et al., 2003). Several studies of genetically variable pathogens such as the human immunodeficiency virus (HIV) and hepatitis C virus have exploited the capacity of the ELISpot to screen for interferon (IFN)-γ responses against diverse human leukocyte antigen (HLA)-restricted epitopes (Malhotra et al., 2007; Lauer et al., 2004). Large scale studies pose particular challenges however; manual steps may be labour intensive and time-consuming over a large number of assays. Maintaining quality control, particularly with respect to precision and accuracy of cytokine detection, depends on a number of technical factors, including cell input numbers, even plating of cells and reagents into wells, peptide concentration, incubation and wash times and the stringency of the spot counting procedure. The added complexity associated with using potentially hundreds of stimulating peptides and multiple clinical samples means that assays need to be highly standardised to minimise inter-assay variability. Finally, manual transcribing of input and output data, manual plating of peptides and multiple clinical samples pose a perennial risk of errors.
We sought to develop an automated system for integrated performance of the ELISpot assay from counting of peripheral blood mononuclear cells (PBMCs) through to electronic capture and archiving of final spot enumeration data. Importantly, ‘automation’ refers to the use of robotics for sample and reagent handling and an integrated information system to electronically track assay performance, protocols and documentation. PBMC preparation is manually performed and the choice and arrangement of peptides into individual plates and assays is configurable by the operator. Here we report the successful implementation of automated ELISpot assays and results of formal quality comparisons between manual and automated systems.
1.1. Automated and manual protocols for enumeration of PBMC
We compared automated and manual protocols performed simultaneously using PBMCs from healthy individuals. Viable cell counts were determined by trypan blue exclusion using a Neubauer haemacytometer or Vi-Cell XR (Beckman Coulter, Sydney, Australia) prior to freezing and following an overnight rest at 37 °C after cryopreservation.
Similar cell counts were obtained for both methods irrespective of using fresh (n=10, manual count: median=66.18, range =19.31 to 114.8; Vi-Cell XR count: median=69, range=22 to 128.1; r=0.99) or cryopreserved cells (n=18, manual count: median=1.60, range=0.61 to 2.41; Vi-Cell XR count: median = 1.41, range = 0.64 to 2.92; r = 0.97) (p=0.23 and p=0.92 for manual versus Vi-Cell XR with fresh and cryopreserved cells respectively, Wilcoxon signed-rank test of paired differences).
1.2. Automation of the ELISpot assay
IFN-γ responses to limiting dilutions (10−7 to 2 μg/mL) of pools of CMV-, EBV- and influenza (CEF)-derived peptides and anti-CD3 antibody (Mabtech, Victoria, Australia) in healthy individuals and additionally HIV-1 Nef-derived peptides (Invi-trogen, Victoria, Australia) in HIV-infected patients were quantified using Mabtech reagents in 96-well nitrocellulose-backed plates (Millipore, Bedford, United States of America). Automated assays were performed using a Biomek FX (Beck-man Coulter) and an integrated ELx 405 washer (BioTek, Vermont, United States of America). The assays were carried out according to the manufacturer’s instructions with the following exceptions; coating anti-IFN-γ antibody was applied at 2 μg/mL and 3, 3′, 5, 5′-tetramethylbenzidine was used for spot detection. Separated PBMCs were counted on a Vi Cell XR anddiluted to 105 PBMCs/well. The Biomek FX software enabled the generation of experimental templates with a graphic interface displaying deck layout, labware and reagents required (Taylor, 2002). The deck set up together with liquid-handling parameters such as the pipetting order and position of reagents in the plate, aspiration and dispensation specifications including mixing, reagent volumes, tip touches and number of washes (Taylor, 2002) were electronically configured by the user and applied to all assays. The number of spots were enumerated on an ELISpot reader (Autoimmun Diagnostika, Strassberg, Germany) and the results were presented as spot forming units (SFUs)/106 PBMCs after subtraction of the background (Karlsson et al., 2003).
1.3. Precision of automated versus manual ELISpot assays
IFN-γ production to limiting dilutions (10−7 to 2 μg/mL) of pools of CEF-derived peptides was measured in six healthy individuals in parallel manual and automated assays. The IFN-γ response was measured in triplicate, replicate mean SFUs/106 PBMCs are presented in Fig. 1. The mean number of SFUs measured by the manual and automated processes were highly correlated for all concentrations greater than 10−7 μg/mL (r>0.83, p<0.05) and no systematic differences in the results between the two process were observed, (p>0.1, Wilcoxon signed-rank test of paired differences). The two processes also had comparable intra-assay coefficients of variation (CVs) across the dilutions (Fig. 1; p=0.3, Wilcoxon signed-rank test of paired differences) with greater relative variation at low concentrations for both processes (Spearman’s correlation between replicate CV and concentration: r=−0.77 manual; r=−0.64 automated; p<0.0001). There was a greater spread of CVs relative to dilutions for manual assays (mean=36.2%, SD=35.2%) compared to the automated process (mean= 27.2%, SD=22.3%), largely due to very high CVs in manual assays at the lowest peptide concentrations.
Fig. 1.
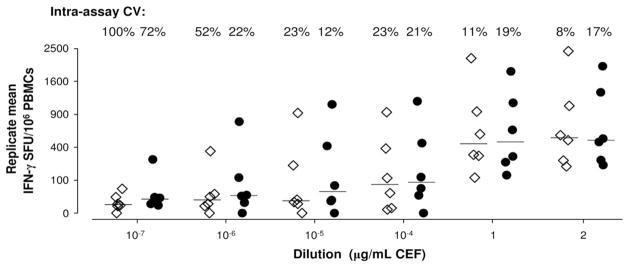
Triplicate mean IFN-γ responses to limiting dilutions of CEF (10−7 to 2 μg/mL) for six healthy controls measured by manual (◇) and automated (●) ELISpot assays. Assay results were comparable (p>0.05 for all dilutions, Wilcoxon signed-rank test of paired differences in triplicate means), with greater variation at weaker dilutions for both processes (Spearman’s correlation between replicate CV and concentration: r=−0.77 manual; r=−0.64 automated; p < 0.0001).
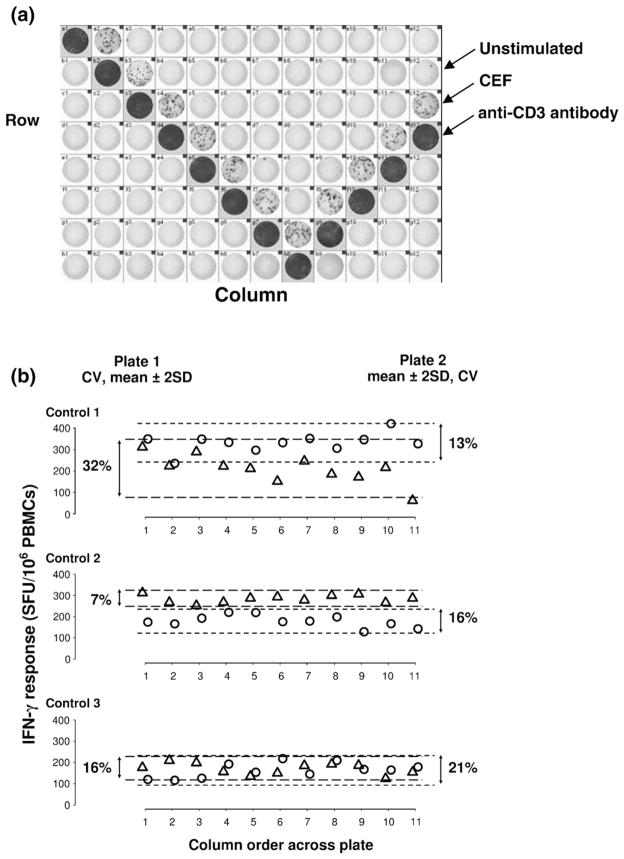
Intra-assay precision and reproducibility of the automated ELISpot was examined further using PBMCs from three healthy individuals tested in duplicate on separate “V” format plates (Fig. 2a). PBMCs were cultured with CEF (2 μg/mL), anti-CD3 antibody (2 μg/mL) and media alone and the number of IFN-γ SFUs/106 PBMCs quantified. Responses to CEF were enumerated in controls 1 and 2 whereas responses to anti-CD3 antibody were enumerated in control 3 due to low CEF responses in this individual (Fig. 2b). No consistent pattern of variability relating to well or row location was observed. Intra-assay variation was in general <20% (mean CV=18%) and the mean inter-assay CV was 23% (Fig. 2b).
Fig. 2.
(a) V-formation of 96-well plates set up to assess plate variation in automated ELISpot assays measuring IFN-γ responses to 2 μg/mL of CEF and anti-CD3 antibody. (b) Responses from Three healthy individuals (plate 1: △, plate 2: ○) to either anti-CD3 (Control 1) or CEF (Controls 2 and 3) chosen according to measurability (range of 100–500 SFUs/106 PBMCs). Intra-assay CVs are provided for each plate, together with dotted lines indicating 2 standard deviations above and below the plate means. The average inter-assay CV was 23%.
1.4. High-throughput testing of HIV-specific responses
The capacity of this optimized system to handle high-throughput processing was investigated by comparing IFN-γ responses across multiple samples to peptide sets that spanned the Nef region of HIV-1. We tested a set of peptides that were each between eight and 11 amino acid residues in length and corresponded to known and predicted optimal HLA class I-restricted CD8+ T-cell epitopes in HIV-1. In addition, we tested longer 15-mer peptides overlapping by 11 amino acids as commonly used in vaccine studies to detect both CD8+ and CD4+ T-cell responses (HIV-1 Consensus Subtype B Nef (15-mer) Peptides; AIDS Research and Reference Reagent Program, NIH, Maryland, United States of America). The T-cell responses were quantified in a cohort of 28 HIV-infected patients over eighty-four 96-well plates. Samples were processed by single operators in batches. Common templates were created, recorded and tracked by the Biomek FX software where the volume, initial location and final destination of sample and reagents were specified; however, templates could also be customised when cell numbers restricted the range of antigens that could be tested. In this way, a single operator could process eight 96-well plates in each run.
As observed in the studies of CEF responses in healthy individuals, precision measured across duplicates using optimal HIV peptides improved with increasing spot number (Spearman’s correlation between duplicate means and CVs: r=−0.35, p<0.0001): 50–150 SFUs, (median=80) CV= 43%,150–300 SFUs, (median=190) CV=32%; 300–500 SFUs, (median = 376) CV = 17%; >500 SFUs, (median = 993) CV=11%. The variation in CVs was more restricted compared to manual assays as described by Maecker et al. (2008) where CVs sharply increased as the mean SFU/2.5×105 PBMCs approached zero. Similar CVs were obtained for the overlapping set of peptides: 50–150 SFUs, (median=83) CV= 37%, 150–300 SFUs, (median=191) CV=21%; >300 SFUs, (median=1319) CV=16%.
2. Conclusions
As the ELISpot assay continues to have widespread applications for large scale field studies, vaccine trials and emerging translation to clinical laboratory investigation, ongoing improvement to the quality and utility of its performance as a high-throughput assay are increasingly important. Here we present a successful and configurable automated system for performance of the ELISpot IFN-γ assay, incorporating automated cell counting and robotics for liquid handling. Integration of the Vi Cell XR, Biomek FX and AID ELISpot plate reader is a critical aspect of this high-throughput system, the advantages of which are shown in Table 1. The presence and magnitude of antigen-specific responses in healthy individuals were detected equally well in the automated system and manual assays however, the precision and reproducibility of the automated ELISpot assay was on average higher and was more stable over varying dilutions of CEF and at low frequency IFN-γ responses and SFUs. Notably, the manual ELISpot is particularly prone to high CVs at lower reagent concentrations which may reflect the inherent variability of biological assays (Aljofan et al., 2008), being further compounded by the small volumes for dilutions. This suggests the use of replicate wells is particularly important for manual assays under low volume conditions. Analysis of the automated system for detecting HIV-specific T-cell responses suggested a generally favourable precision range associated with automation compared with other manual studies.
Table 1.
Characteristics of the automated ELISpot compared with the manual process.
| Automated ELISpot assay | Manual ELISpot assay | |
|---|---|---|
| Throughput | High | Limited |
| Precision/reproducibility | Mean CV=27.2%, SD=22.3%* | Mean CV=36.2%, SD=35.2%* |
| No plate location effects | More subject to high CV at low SFUs | |
| Sample handling | Minimal | Labour intensive |
| Plate design and documentation | Automatically recorded and trackable | Operator dependent |
CVs measured in this study using anti-CEF responses in healthy subjects.
Automation ensured complete standardisation in assay performance across many samples, increased output, decreased sample handling and reduced user time, all of which are necessary for translation of ELISpot-based assays to clinical laboratory practice. Unlike manual systems, data integrity is secured by the generation of electronic files recording all aspects of the assay in real time. Thus the identity of every reagent and every sample in every well and plate at any point in time, can be configured, tracked and quality-assured in large scale projects handling hundreds of assays and clinical samples.
Acknowledgments
The authors wish to thank participants of the Western Australian HIV Cohort Study and colleagues in the Centre for Clinical Immunology and Biomedical Statistics (CCIBS). This research is supported by the Australian National Health and Medical Research Council (Program grant ID 384702) and the Bill and Melinda Gates Foundation.
Abbreviations
- ELISpot
enzyme linked immunospot
- HIV
human immunodeficiency virus
- IFN
interferon
- PBMCs
peripheral blood mono-nuclear cells
- CEF
pools of CMV-, EBV- and influenza-derived peptides
- CV
coefficient of variation
- SFUs
spot forming units
- HLA
human leukocyte antigen
References
- Aljofan M, Porotto M, Moscona A, Mungall BA. Development and validation of a chemiluminescent immunodetection assay amenable to high throughput screening of antiviral drugs for Nipah and Hendra virus. J Virol Methods. 2008;149:12. doi: 10.1016/j.jviromet.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Ferrari G, Janetzki S. Measurement of cytokine release at the single cell level using the ELISPOT assay. Methods. 2006;38:274. doi: 10.1016/j.ymeth.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Karlsson AC, Martin JN, Younger SR, Bredt BM, Epling L, Ronquillo R, Varma A, Deeks SG, McCune JM, Nixon DF, Sinclair E. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J Immunol Methods. 2003;283:141. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kreher CR, Dittrich MT, Guerkov R, Boehm BO, Tary-Lehmann M. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J Immunol Methods. 2003;278:79. doi: 10.1016/s0022-1759(03)00226-6. [DOI] [PubMed] [Google Scholar]
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131:1898. doi: 10.1378/chest.06-2471. [DOI] [PubMed] [Google Scholar]
- Lauer GM, Barnes E, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Robbins GK, Casson DR, Reiser M, Dusheiko G, Allen TM, Chung RT, Walker BD, Klenerman P. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004;127:924. doi: 10.1053/j.gastro.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Hassler J, Payne JK, Summers A, Comatas K, Ghanayem M, Morse MA, Clay TM, Lyerly HK, Bhatia S, Ghanekar SA, Maino VC, Delarosa C, Disis ML. Precision and linearity targets for validation of an IFNgamma ELISPOT, cytokine flow cytometry, and tetramer assay using CMV peptides. BMC Immunol. 2008;9:9. doi: 10.1186/1471-2172-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra U, Li F, Nolin J, Allison M, Zhao H, Mullins JI, Self S, McElrath MJ. Enhanced detection of human immunodeficiency virus type 1 (HIV-1) Nef-specific T cells recognizing multiple variants in early HIV-1 infection. J Virol. 2007;81:5225. doi: 10.1128/JVI.02564-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PB. Optimizing assays for automated platforms. Mod Drug Discov. 2002;5:37. [Google Scholar]