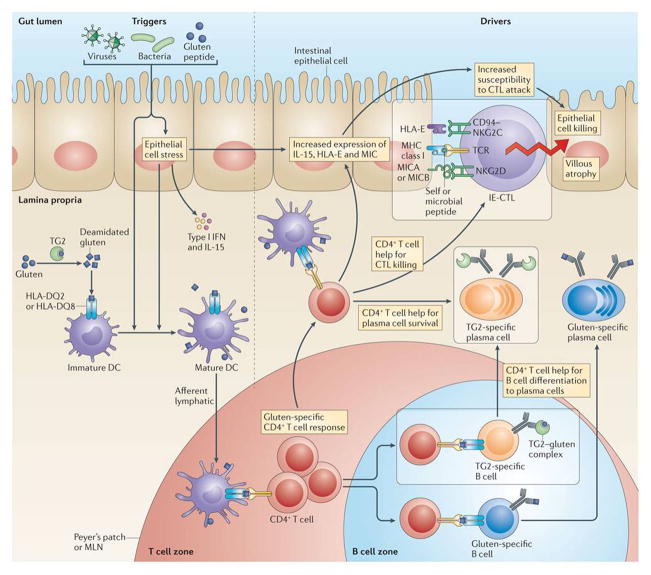
Figure 3. Dietary antigen drives autoimmune processes in coeliac disease.
Triggers such as viruses, pathogens and pathobionts activate antigen-presenting cells and epithelial cells. Antigen-presenting cells acquire proinflammatory properties and present gluten to induce the activation of gluten-specific HLA-DQ2- or HLA-DQ8-restricted CD4+ T cells. Because transglutaminase 2 (TG2) and gluten form complexes, TG2-specific autoreactive B cells internalize TG2–gluten complexes and present gluten peptides on HLA-DQ2 or HLA-DQ8 at their surface. Gluten-specific B cells can bind and present deamidated gluten peptides in a conventional manner. The gluten-specific CD4+ T cells provide help to both autoreactive TG2-specific and gluten-specific B cells, which will differentiate into antibody producing plasma cells. Activated gluten-specific CD4+ T cells also provide signals (that remain to be fully defined) to pre-activated epithelial cells, which upregulate IL-15 and non-classical MHC class I molecules. Consequently, intraepithelial cytotoxic T lymphocytes (IE-CTLs) acquire lymphokine activated killer activity and a decreased T cell receptor (TCR) activation threshold and will kill epithelial cells based on the recognition of stress signals. Whether IE-CTLs with a decreased TCR activation threshold will recognise low affinity epithelial antigens and antigens of the microbiota through their TCR remains to be determined. The autoimmune phenomena are boxed.