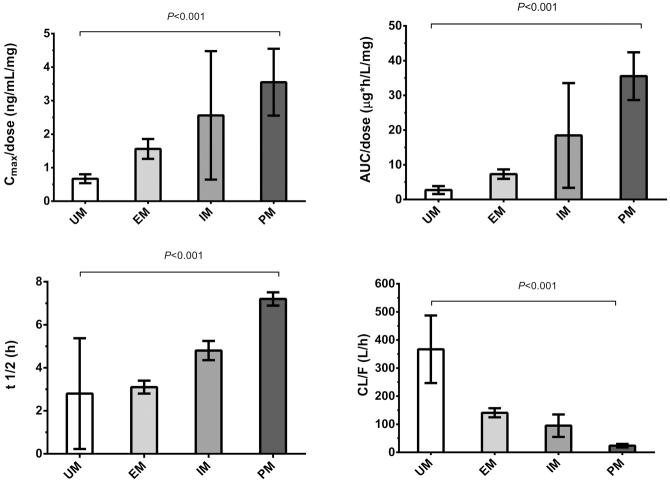
Figure 2. A-D. Effects of CYP2D6 metabolizer phenotype on selected pharmacokinetic parameters.
A Peak dose-normalized plasma metoprolol concentration (Cmax/dose); B Dose-normalized area under the plasma concentration (AUC/dose); C Elimination half-life (t1/2); D Apparent oral clearance (CL/F) The difference between ultra-rapid metabolizers and poor metabolizers in all four parameters is highly statistically significant (all p<0.001). Error bars represent standard deviations.