Table 2.
Use of Pol–PPh3/I2 for synthesis of amides and Weinreb amides.[a]
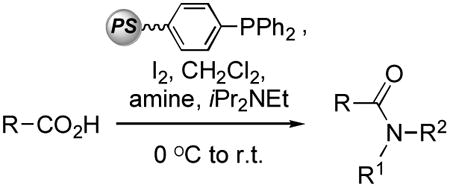
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Acid | Amine | Time | Yield |
| 1 |
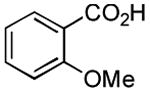
|
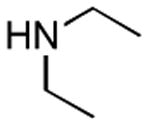
|
2 h | 75%[21] |
| 2 |
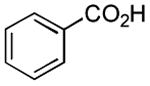
|
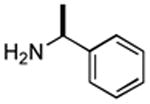
|
2 h | 72%[25] |
| 3 |
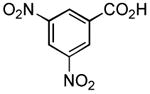
|
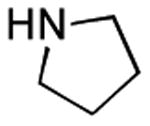
|
1 h | 65% |
| 4 |
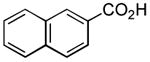
|
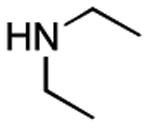
|
11 h 3 h |
57%[20] 71%[b] |
| 5 |
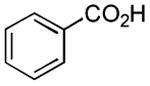
|

|
6 h | 53%[41] |
| 6 |
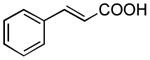
|
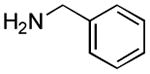
|
50 min 1 h |
53%[42] 78%[b] |
| 7 |
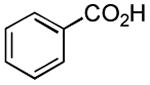
|
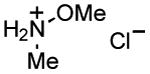
|
1 h | 76%[32,c] |
| 8 |
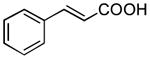
|
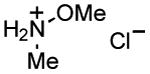
|
1 h | 79%[39h,c] |
Reactions were conducted using 1 mmol each of Pol–PPh3 (2.28 mmol/g loading), I2 carboxylic acid, amine, and 1.5 mmol of iPr2NEt in 4 mL of CH2Cl2, unless specified otherwise.
Yield obtained by using 2 molar equiv each of Pol–PPh3 and I2.
2.5 Molar equiv of iPr2NEt was used.
