Abstract
Background
Matrix metalloproteinases (MMPs) have been implicated in HIV associated neurological injury; however, this relationship has not been studied early in infection.
Methods
Plasma levels of MMP-1, -2, -7, -9, and -10 measured using Luminex technology were compared in 52 HIV and 21 seronegative participants of the Chicago Early HIV Infection study. MMP levels were also examined in HIV subgroups defined by antibody reactivity, viremia, and antiretroviral status, as well as in available CSF samples (n=9). MMPs were evaluated for patterns of relationship to cognitive function and to quantitative magnetic resonance measurements of the brain derived in vivo.
Results
Plasma MMP-2 levels were significantly reduced in early HIV infection and correlated with altered white matter integrity and atrophic brain changes. MMP-9 levels were higher in the treated than naïve HIV subgroup. Only MMP-2 and -9 were detected in CSF; CSF MMP-2 correlated with white matter integrity and with volumetric changes in basal ganglia. Relationships with cognitive function were also identified.
Conclusions
MMP-2 levels in plasma and in CSF correspond to early changes in brain structure and function. These findings establish a link between MMPs and neurological status previously unidentified in early HIV infection.
Keywords: Matrix metalloproteinases, Acute HIV, diffusion tensor imaging, neuro-AIDS, HIV-associated neurocognitive disorder
Introduction
HIV infection results in serious central nervous system injury and cognitive deterioration in many patients. Neurological injury has been ascribed to deleterious effects of unrelenting immune activation (McArthur et al, 2005). Matrix metalloproteinases (MMPs) have been investigated in this setting (Conant et al, 1999; Liuzzi et al, 2000; Louboutin et al, 2010; Ragin et al, 2009; Van Lint and Libert, 2007) because of their involvement in regulation of neuroinflammation (McQuibban et al, 2002), blood-brain barrier permeability (Louboutin et al, 2010), and cell migration (Agrawal et al, 2008; Sternlicht and Werb, 2001). MMPs are extracellular proteases that may facilitate infiltration of the central nervous system by infected monocytes by influencing blood-brain barrier permeability (Louboutin et al, 2010; Van Lint and Libert, 2007). MMP activity is elevated in cerebrospinal fluid (CSF) of infected patients (Conant et al, 1999; Liuzzi et al, 2000), and plasma MMP levels correlate with the severity of brain atrophy quantified in vivo in advanced infection (Ragin et al, 2011; Ragin et al, 2009). MMPs may be of particular relevance in early infection. Viral invasion of the brain occurs soon after initial infection (Chiodi et al, 1988; Davis et al, 1992), causing neuroinflammation and potentially establishing a viral reservoir. MMP levels and relationships to brain changes in this period, however, are largely unknown.
This study determined plasma levels of MMP-1 (collagenase-1), MMP-2 (gelatinase A), MMP-7 (matrilysin), MMP-9 (gelatinase B), and MMP-10 (stromelysin-2) in participants of the Chicago Early HIV Infection study, a cohort (n=52) infected on average less than one year. This analysis is also distinguished by inclusion of an age matched seronegative control group (n=21); information concerning normative plasma MMP levels is very limited. MMPs were also determined in HIV subgroups based on antibody reactivity (non-reactive and reactive), antiretroviral treatment status (ARV and naïve), and viremia (uncontrolled and controlled, i.e. viral load greater than 50 copies/mL). MMP levels in CSF, available for 9 of the infected subjects, were also examined. To evaluate MMP relationships with neurological status, brain volumetric measurements were derived using high resolution neuroanatomic imaging. Diffusion Tensor Imaging (DTI) was used to assess microstructural alterations (Le Bihan, 2003). DTI parameters include fractional anisotropy (FA), which is higher in intact axons and systematically reduced with loss of white matter integrity, and mean diffusivity (MD), which is sensitive to microstructural changes that increase (e.g. atrophy) or decrease (e.g. edema) overall molecular diffusion. MMPs were also evaluated for relationships to clinical status measures and cognitive function measured by a comprehensive neuropsychological evaluation.
Methods
Participants
For enrollment in the Chicago Early HIV Infection cohort study, individuals with self-reported HIV infection were evaluated for likelihood of recent infection based on either a prior negative test result or compelling available information concerning probable time of initial viral exposure. Exclusion criteria for study entry included history of chronic neurological disorder, head injury, radiation or chemotherapy in prior 30 days, uncontrolled seizure disorders, experimental drugs or any vaccination within past 15 days, inability to understand due to mental condition, chronic or active alcohol abuse, chronic or active drug abuse, pregnancy, opportunistic infection, cancer, other medical condition (heart, liver or kidney) and magnetic resonance (MR) contraindication (metal implants or claustrophobia). The Institutional Review Board of Northwestern University approved this investigation, and all subjects signed an informed consent document.
Clinical Status
Blood samples were collected from all subjects. Serostatus was determined by ELISA and Western blot. Clinical measures for HIV subjects included CD4+ cell count, CD8+ cell count, CD4/CD8 ratio, hemoglobin, and plasma HIV RNA copies/mL (viral load). CSF samples were collected from 9 HIV subjects consenting to lumbar puncture. To assess relative recency of infection, samples from the HIV subjects were also analyzed using an early infection assay (EIA) (Blood Systems Research Institute, San Francisco) designed to evaluate individuals whose antibody response against the virus is still evolving (Keating et al, 2012). For the HIV group (n = 52), the mean estimated period from initial infection was less than one year. Thirteen of these subjects were antibody nonreactive and conservatively estimated to be infected less than 70 days. In the HIV group, absolute CD4+ cell counts ranged from 139 to 1,282/mm3 with mean of 548 ± 252/mm3 and median of 509/mm3. Log (base 10) plasma viral load (copies/mL) ranged from undetectable to 5.54 and mean of 3.18 ± 1.34. Twenty seven HIV subjects were treatment naïve. Of those receiving treatment, the majority were on efavirenz/tenofovir/emtricitabine (trade name Atripla).
MMP Luminex Assays
Matrix metalloproteinase profiles were measured in plasma for all subjects and in CSF for consenting HIV subjects. Samples were assayed using the MILLIPLEX MAP Human MMP Panel 2 (Millipore) including MMP-1, MMP-2, MMP-7, MMP-9, and MMP-10 using a 1:20 dilution and following the manufacturer’s protocols. MMP-1, -2, -7, and -10 antibodies bind to both pro- and active forms of the molecules. Because the antibodies used are proprietary, the exact binding specificity for MMP-9 is unavailable. The lower limits of detection for MMPs were 54 pg/mL, 468.4 pg/mL, 965 pg/mL, 24.7 pg/mL and 49.1pg/mL, respectively. Standard curves were run in duplicate wells on each plate using reagents provided by the manufacturer. Samples were run in duplicate, acquired and analyzed on a Labscan 100 analyzer (Luminex) using Bio-Plex manager 6.0 software (Bio-Rad). Each run included internal and external controls, all quality control reagents were within range, and co-efficient of variation (CV%) was an average of 14% for all controls.
MRI Analysis
Radiological variables included volumetric and DTI measurements derived in vivo for major brain tissue classes and specific neuroanatomic structures. Brain regions were segmented based on high resolution 3T MPRAGE images and automated algorithms including SIENAX and Freesurfer. A novel automated “autoregional” 3D volume of interest image analysis strategy (Wu et al, 2012) was used to derive DTI parameters, including fractional anisotropy and mean diffusivity. Details are presented in Supplementary Methods.
Neuropsychological Assessments
All participants were evaluated with a neuropsychological test battery that assessed motor skills, cognitive flexibility, abstraction, verbal memory, visual memory, and visuoconstructional skills. Details and references are in Supplementary Methods.
Statistical Methods
Primary variables for analysis included plasma MMP-1, -2, -7, -9, and -10 levels for all subjects. CSF MMP-2 and -9 levels were available for 9 consenting HIV subjects. (Levels of CSF MMP-1, -7, and -10 were undetectable for all 9 samples). Distributional assumptions were evaluated prior to analysis. MMP group comparisons were accomplished using t-tests or Mann-Whitney tests. For comparisons involving three subgroups, ANOVA or Kruskal-Wallis tests were used. Pearson or Spearman correlation coefficients were used to determine relationships between MMPs and the imaging, clinical, and neuropsychological variables. Missing variables were excluded pairwise for all analyses. A significance level of 0.05 was used for a priori analyses. The Bonferroni correction (0.05/n) was used to correct for multiplicity. Analyses were executed using IBM SPSS 20.0.0 (Chicago, IL).
Results
This study included 52 HIV seropositive (46 Males, 6 Females; mean age 33.2 ± 9.9) and 21 seronegative (16 Males, 5 Females; mean age 31.4 ± 8.8) participants (Table 1). HIV and control groups did not differ in age (p = 0.471), gender (p = 0.188), race (p = 0.317), or education (p = 0.282). North American Adult Reading test score, a measure of general intellectual performance (Blair and Spreen, 1989), also did not differ (p = 0.072). Clinical characteristics of the HIV group are presented in Table 2.
Table 1.
Demographics of the Chicago Early HIV Cohort
| Characteristics | HIV (n=52) | Controls (n=21) | p-value |
|---|---|---|---|
| Age (Years) | 33.2 ± 9.9 | 31.4 ± 8.8 | 0.471 |
| Gender (% Male) | 88.5% | 76.2% | 0.188 |
| Race (% White) | 62.7% | 76.2% | 0.317 |
| Education (% Attended College) | 75.0% | 89.5% | 0.282 |
| NART-R Scorea | 33.9 ± 12.6 | 39.8 ± 11.0 | 0.072 |
NART-R: North American Adult Reading Test
Table 2.
Clinical Characteristics of the HIV Group
| Variables | Mean ± SD | Range |
|---|---|---|
| CD4+ cell count | 548 ± 252 | 139 - 1,282 |
| CD8+ cell count | 885 ± 442 | 276 - 1,845 |
| CD4/CD8 ratio | 0.72 ± 0.35 | 0.22 - 1.73 |
| EIAa Score | 32.9 ± 23.7 | 0.13 - 80.50 |
| Plasma HIV RNA | ||
| Log10 Copies/mL | 3.18 ± 1.34 | 0b - 5.54 |
| % Aviremicb | 22.4% | |
| ARVc (% Naïve) | 51.9% |
EIA: Early Infection Assay.
Aviremic: <50 copies/mL
ARV: Antiretroviral therapy
Plasma MMP-2 levels were reduced in the HIV compared to the control group (p < 0.001) (Table 3). In further analysis, MMP-2 also differed in HIV antibody reactive/non-reactive subgroups and controls (p < 0.001; Table 3). The reduction in MMP-2 levels was more pronounced in HIV subjects who had developed an antibody response than in the most recently infected (antibody nonreactive) subjects (Fig 1).
Table 3.
MMP levels by group
| Plasma | HIV | Control (Group 0) | t-test | CSF | HIV |
|---|---|---|---|---|---|
|
| |||||
| n=52 | n=21 | p-value | n=9 | ||
| MMP-1 | 1,179 ± 925 | 1,013 ± 627 | 0.457 | MMP-1 | Undetectable |
| MMP-2 | 41,740 ± 13,123 | 58,800 ± 13,970 | <0.001** | MMP-2 | 6,700 ± 2,297 |
| MMP-7 | 4,989 ± 2,348 | 4,415 ± 1,731 | 0.318 | MMP-7 | Undetectable |
| MMP-9a | 41,606 ± 38,926 | 33,385 ± 16,230 | 0.958 | MMP-9 | 345 ± 375 |
| MMP-10 | 544 ± 342 | 471 ± 123 | 0.347 | MMP-10 | Undetectable |
| Subgroups | Group 1 | Group 2 | ANOVA | Planned t-tests (p-value) | ||
|---|---|---|---|---|---|---|
| EIA Group | Nonreactive (n=13) | Reactive (n=39) | p-value | 0/1 | 0/2 | 1/2 |
| MMP-1 | 1,335 ± 1,060 | 1,138 ± 898 | 0.614 | 0.296 | 0.575 | 0.553 |
| MMP-2 | 49,675 ± 12,659 | 39,653 ± 12,582 | <0.001** | 0.091† | <0.001** | 0.030* |
| MMP-7 | 5,952 ± 2,757 | 4,737 ± 2,200 | 0.178 | 0.067† | 0.566 | 0.147 |
| MMP-9a | 47,565 ± 65,512 | 40,038 ± 29,474 | 0.769 | 0.583 | 0.788 | 0.493 |
| MMP-10 | 556 ± 299 | 540 ± 356 | 0.638 | 0.406 | 0.391 | 0.901 |
| ARV Status | Naïve (n=27) | Initiated (n=25) | p-value | 0/1 | 0/2 | 1/2 |
| MMP-1 | 1,079 ± 752 | 1,279 ± 1,079 | 0.547 | 0.753 | 0.328 | 0.461 |
| MMP-2 | 45,164 ± 13,226 | 38,318 ± 12,351 | <0.001** | 0.002** | <0.001** | 0.070† |
| MMP-7 | 4,747 ± 2,384 | 5,232 ± 2,337 | 0.455 | 0.600 | 0.198 | 0.480 |
| MMP-9a | 35,073 ± 43,245 | 48,139 ± 33,723 | 0.050* | 0.246 | 0.211 | 0.016* |
| MMP-10 | 559 ± 311 | 529 ± 377 | 0.606 | 0.212 | 0.484 | 0.762 |
MMP measurements are in units of pg/mL.
CSF: Cerebrospinal fluid; EIA: Early Infection Assay; ARV: Antiretroviral.
Kruskal-Wallis and Mann-Whitney tests used for MMP-9.
Significant at the 0.01 level.
Significant at the 0.05 level.
Nearly significant (p < 0.10).
Fig. 1.
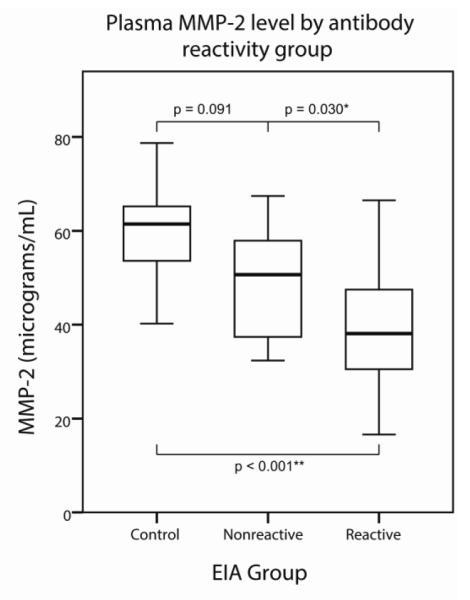
Boxes represent interquartile range (IQR). Whiskers represent data points within 1.5 IQRs of the box. A significant downward linear trend was seen for plasma MMP-2 with respect to HIV progression as grouped by antibody reactivity (p < 0.001; deviation p = 0.921). Mean MMP-2 level was reduced in the HIV antibody reactive group compared to the nonreactive group (p = 0.030). When compared to the control group, MMP-2 levels differed significantly for the antibody-reactive HIV subgroup (p < 0.001) and approached significance for the very early, nonreactive HIV subgroup (p = 0.091)
Comparison of MMP levels in ARV-naïve, ARV-initiated, and control groups indicated significant differences for MMP-2 (p < 0.001) and for MMP-9 (p = 0.050) (Table 3). MMP-9 levels were significantly higher in the ARV-initiated than -naïve subgroup (p = 0.016). MMP-2 showed reductions in both ARV-naïve and -initiated groups compared to controls (p = 0.002; p < 0.001). Further analysis (Table S1) indicated that MMP-2 levels did not differ between viremic and aviremic HIV subgroups and were reduced in both HIV subgroups (p < 0.001; p < 0.001) compared to controls.
Table 4 presents MMP correlations with Freesurfer volumetric measurements of localized brain regions and landmarks, including results significant at both uncorrected (p < 0.05) and Bonferroni corrected (p < 0.003) levels. Plasma MMP-1 was significantly correlated with volumetric measurements of cerebral white matter (p = 0.036), brain stem (p = 0.016), and fourth ventricle (p = 0.005). MMP-7 was correlated with amygdala (p = 0.034) and cerebellum cortex (p = 0.045). MMP-9 was correlated with splenium (p = 0.013) and fourth ventricle (p = 0.035). MMP-10 was correlated with cerebral white matter (p = 0.031) and fourth ventricle (p = 0.037). CSF MMP-2 was correlated with caudate (p = 0.036), putamen (p = 0.030), accumbens (p = 0.020) and pallidum (p = 0.002) volumes. CSF MMP-9 was correlated with inferior lateral ventricle (p = 0.050). No significant correlations were identified with SIENAX measurements of the major brain constituent tissue classes (i.e. total gray matter, white matter or CSF) (Table S2).
Table 4.
MMP Relationships with Brain Volumetric Measurements
| Plasma (n=68) | CSF (n=9) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Brain Region | MMP-1 | MMP-2 | MMP-7 | MMP-9 | MMP-10 | MMP-2 | MMP-9 |
| Cerebral Cortex | 0.071 | 0.230† | −0.086 | −0.005 | −0.200 | −0.517 | 0.117 |
| Cerebral WM | −0.259* | −0.084 | 0.010 | 0.107 | −0.265* | 0.083 | −0.317 |
| Cerebellum Cortex | 0.059 | 0.025 | −0.247* | 0.096 | −0.036 | 0.017 | 0.150 |
| Cerebellum WM | −0.135 | 0.137 | 0.017 | −0.103 | −0.105 | 0.200 | −0.267 |
| Corpus Callosum | −0.232 | 0.034 | 0.030 | 0.092 | 0.120 | −0.401 | −0.413 |
| Caudate | −0.170 | −0.198 | −0.085 | −0.065 | −0.217† | −0.700* | 0.067 |
| Putamen | 0.076 | −0.148 | 0.009 | 0.007 | −0.137 | −0.717* | 0.100 |
| Pallidum | 0.004 | −0.043 | 0.014 | −0.068 | 0.026 | − 0.883 ** | −0.133 |
| Accumbens | 0.052 | −0.101 | −0.147 | 0.060 | −0.166 | −0.750* | −0.150 |
| Amygdala | 0.066 | −0.003 | −0.262* | 0.095 | −0.222† | 0.017 | 0.133 |
| Hippocampus | 0.042 | 0.073 | −0.041 | 0.123 | −0.011 | −0.433 | −0.317 |
| Ventral Diencephalon | −0.233† | 0.028 | 0.073 | −0.106 | 0.060 | −0.233 | −0.050 |
| Thalamus | −0.061 | 0.014 | 0.064 | −0.182 | −0.082 | −0.483 | −0.317 |
| Brain Stem | −0.296* | 0.072 | 0.080 | −0.084 | 0.037 | 0.000 | −0.400 |
| Third Ventricle | −0.091 | −0.148 | 0.058 | 0.080 | −0.090 | 0.267 | −0.317 |
| Fourth Ventricle | −0.345* | −0.146 | −0.007 | −0.260* | −0.258* | −0.233 | 0.450 |
| Inf Lateral Ventricles | −0.149 | 0.054 | 0.134 | 0.055 | 0.015 | −0.200 | −0.667* |
| Lateral Ventricles | 0.030 | −0.093 | 0.079 | 0.168 | −0.130 | 0.050 | −0.117 |
Pearson correlation coefficients (Spearman used for MMP-9 and CSF).
Lateralized regions have been summed for left and right hemisphere; WM: White matter
Bold correlation coefficient: Significant at the Bonferroni corrected level of 0.05/18.
Significant at the 0.01 level.
Significant at the 0.05 level.
Significant at the 0.10 level.
MMPs were also correlated with autoregionally determined DTI parameters (Hutten et al, 2011; Wu et al, 2012). The studied microstructural measures included fractional anisotropy (FA), which reflects loss of white matter integrity, and mean diffusivity (MD), which is sensitive to microstructural changes that increase (e.g. atrophy) or decrease (e.g. edema) diffusion (Table 5). Regions in italics indicate significant correlations at the Bonferroni corrected level of 0.05/9. For fractional anisotropy (FA), MMP-1 was correlated with corpus callosum (p = 0.037), cerebral white matter (p = 0.010), and hippocampus (p = 0.048) anisotropy. MMP-2 was correlated with cerebral white matter (p = 0.013) and whole brain white matter (p = 0.003) anisotropy. CSF MMP-2 was correlated with cerebral white matter (p = 0.031) and corpus callosum (p = 0.023) anisotropy. For mean diffusivity (MD), MMP-2 was correlated with whole brain (p = 0.001), cerebral cortex (p = 0.001), cerebral white matter (p = 0.002), putamen (p = 0.013), thalamus (p = 0.023), and whole brain white matter (p = 0.004) diffusivity. MMP-7 was correlated with whole brain (p = 0.010), caudate (p = 0.012), and putamen (p = 0.020) diffusivity. MMP-10 was correlated with hippocampus diffusivity (p = 0.031). CSF MMP-9 was correlated with caudate diffusivity (p = 0.024).
Table 5.
MMP Relationships with Autoregional DTI Brain Measurements
| Plasma (n=69) | CSF (n=9) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Fractional Anisotropy (FA) | MMP-1 | MMP-2 | MMP-7 | MMP-9 | MMP-10 | MMP-2 | MMP-9 |
| Whole Brain | −0.140 | 0.053 | 0.220† | −0.040 | −0.103 | −0.441 | −0.380 |
| Whole Brain WM | −0.207† | 0.356 ** | 0.146 | −0.112 | −0.004 | −0.561 | −0.337 |
| Cerebral Cortex | 0.031 | −0.148 | 0.018 | −0.035 | −0.068 | 0.417 | 0.234 |
| Cerebral WM | −0.309** | 0.298* | 0.073 | −0.165 | 0.007 | −0.714* | −0.274 |
| Corpus Callosum | −0.254* | 0.053 | 0.033 | 0.004 | −0.026 | −0.739* | −0.445 |
| Caudate | −0.086 | 0.019 | 0.250* | −0.144 | 0.069 | 0.629 | 0.502 |
| Putamen | 0.139 | −0.061 | 0.140 | −0.030 | −0.051 | 0.512 | 0.037 |
| Hippocampus | 0.239* | −0.155 | 0.057 | −0.146 | 0.152 | −0.438 | −0.143 |
| Thalamus | −0.059 | 0.165 | 0.177 | −0.003 | −0.086 | −0.389 | −0.328 |
|
| |||||||
| Mean Diffusivity (MD) | MMP-1 | MMP-2 | MMP-7 | MMP-9 | MMP-10 | MMP-2 | MMP-9 |
|
| |||||||
| Whole Brain | −0.153 | − 0.398 ** | −0.307* | 0.077 | −0.030 | −0.251 | −0.617† |
| Whole Brain WM | 0.002 | − 0.340 ** | −0.088 | 0.171 | −0.094 | 0.234 | −0.136 |
| Cerebral Cortex | 0.073 | − 0.392 ** | −0.209† | 0.145 | −0.157 | 0.505 | −0.143 |
| Cerebral WM | 0.061 | − 0.363 ** | −0.087 | 0.187 | −0.119 | 0.459 | −0.146 |
| Corpus Callosum | −0.017 | 0.086 | 0.199 | −0.097 | −0.018 | −0.133 | −0.339 |
| Caudate | −0.042 | 0.142 | 0.301* | −0.036 | 0.137 | −0.237 | −0.736* |
| Putamen | −0.065 | −0.299* | −0.279* | 0.172 | −0.053 | −0.007 | 0.189 |
| Hippocampus | −0.222† | 0.016 | −0.208† | 0.015 | −0.250* | 0.227 | 0.108 |
| Thalamus | −0.090 | −0.274* | −0.220† | 0.059 | −0.095 | −0.110 | 0.502 |
Pearson correlation coefficients (Spearman used for MMP-9 and CSF).
Lateralized regions have been averaged for left and right hemisphere; WM: White matter
Bold correlation coefficient: Significant at the Bonferroni corrected level of 0.05/9.
Significant at the 0.01 level.
Significant at the 0.05 level.
Nearly significant (p < 0.10).
Table 6 presents correlations with clinical measures in HIV subjects. MMP-2 was correlated with CD4/CD8 ratio (p = 0.049). MMP-7 was correlated with viral load (p = 0.044). CSF MMP-2 was correlated with CD8+ cell count (p = 0.007). Neuropsychological correlations are presented in Table 7. MMP-1 was correlated with Rey auditory verbal memory (p = 0.019), letter-number sequencing (p = 0.019), and trail-making performance (p = 0.003). MMP-7 was correlated with timed gait (p = 0.003). MMP-10 was correlated with trail-making (p = 0.035). CSF MMP-2 was correlated with grooved pegboard performance (p = 0.049). CSF MMP-9 was correlated with verbal fluency (p = 0.042). Neuropsychological correlations failed to meet more conservative Bonferroni criteria.
Table 6.
Correlations of MMPs with HIV clinical variables
| Plasma (n=48) | CSF (n=9) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Clinical Variables | MMP-1 | MMP-2 | MMP-7 | MMP-9 | MMP-10 | MMP-2 | MMP-9 |
| CD4+ cell count | −0.154 | 0.052 | 0.107 | 0.234 | −0.061 | −0.400 | −0.333 |
| CD8+ cell count | −0.278† | −0.228 | −0.171 | 0.083 | 0.048 | −0.817** | −0.117 |
| CD4/CD8 ratio | 0.144 | 0.285* | 0.218 | 0.096 | −0.144 | 0.427 | −0.326 |
| HIV RNA (viral load) | 0.036 | −0.054 | −0.298* | −0.199 | 0.006 | 0.100 | 0.267 |
| Hemoglobin | 0.047 | −0.181 | 0.068 | 0.105 | −0.101 | 0.433 | −0.417 |
Pearson correlation coefficients (Spearman used for MMP-9 and CSF).
Significant at the 0.01 level.
Significant at the 0.05 level.
Nearly significant (p < 0.10).
Table 7.
Correlations of MMPs with cognitive status measures
| Plasma (n=69) | CSF (n=9) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cognitive Measures | MMP-1 | MMP-2 | MMP-7 | MMP-9 | MMP-10 | MMP-2 | MMP-9 |
| Verbal Memory | |||||||
| RAVLT | −0.283* | 0.101 | −0.059 | −0.010 | −0.039 | −0.311 | −0.664† |
| LNS | −0.286* | 0.235† | −0.061 | 0.067 | −0.011 | 0.238 | −0.204 |
| Visual Memory | |||||||
| ROCF Recall | −0.200† | 0.175 | −0.108 | 0.086 | 0.021 | −0.610 | −0.464 |
| Visuoconstruction | |||||||
| ROCF Copy | −0.218† | 0.240† | 0.007 | −0.119 | 0.082 | −0.325 | 0.012 |
| Frontal Executive | |||||||
| Verbal Fluency | −0.009 | 0.223† | −0.159 | 0.149 | 0.015 | 0.233 | −0.683* |
| Odd Man Out | −0.205† | 0.064 | 0.014 | −0.125 | −0.083 | −0.186 | −0.319 |
| Trail-making | 0.350** | −0.084 | 0.084 | −0.142 | 0.254* | 0.201 | 0.435 |
| Psychomotor | |||||||
| Digit Symbol | −0.175 | 0.079 | −0.167 | 0.208† | −0.110 | −0.075 | 0.209 |
| CALCAP Choice | 0.100 | −0.005 | 0.013 | −0.184 | −0.157 | 0.444 | −0.184 |
| CALCAP Sequential | 0.072 | 0.061 | −0.056 | −0.134 | −0.061 | 0.100 | −0.276 |
| Motor Speed | |||||||
| Grooved Pegboard | 0.127 | −0.038 | 0.111 | 0.042 | 0.128 | −0.669* | 0.351 |
| Timed Gait | −0.138 | 0.091 | 0.359** | −0.010 | 0.033 | −0.305 | −0.322 |
Pearson correlation coefficients (Spearman used for MMP-9 and CSF).
RAVLT: Rey Auditory Verbal Learning Test; ROCF: Rey-Osterrieth Complex Figure; CALCAP: California Computerized Assessment Package; LNS: Letter Number Sequencing
Bold correlation coefficient: Significant at the Bonferroni corrected level of 0.05/18.
Significant at the 0.01 level.
Significant at the 0.05 level.
Nearly significant (p < 0.10).
Discussion
This study analyzed MMP-1, -2, -7, -9, and -10 levels in an early HIV infection cohort and in an age-matched control group. Of these, MMP-2 showed the most prominent relationship with brain status in early HIV infection. Circulating MMP-2 levels were reduced in the early HIV group compared to controls (Table 3) and were correlated with DTI measures (fractional anisotropy and mean diffusivity) reflecting microstructural brain alterations (Table 5). Specific correlates of plasma MMP-2 included loss of white matter integrity (reduced anisotropy), as well as atrophic alterations (increased diffusivity) in whole brain, in brain white matter, cerebral cortex, and cerebral white matter. MMP-2 relationships with loss of cerebral white matter integrity and with increased diffusivity in putamen and thalamus were also observed. Notably, correlates of MMP-2 mirror brain regions vulnerable to injury in HIV infection. Autopsy and in vivo imaging studies in HIV infection have shown similar abnormalities in white matter and basal ganglia, as well as abject brain atrophy (Chen et al, 2009; Filippi et al, 2001; Hutten et al, 2011; Navia et al, 1986; Thompson et al, 2005; Wohlschlaeger et al, 2009).
Analysis of HIV subgroups suggests that plasma MMP-2 levels decline over the course of HIV. Plasma MMP-2 reduction was more marked in the antibody reactive than in the early nonreactive HIV subgroup compared to controls (Fig 1). In addition, plasma MMP-2 levels correlated with CD4/CD8 ratio (Table 6), a prognostic marker of systemic HIV progression (Taylor et al, 1989). The relative plasma MMP-2 reduction was not mitigated by antiretrovirals; levels were more markedly reduced in ARV treated than naïve subgroups, despite similar duration of infection (Table 4). ARV response for certain patients may have been limited because of the early infection period and short duration of treatment. To account for this, viremia status (controlled vs. uncontrolled) was analyzed, supporting the results for the treatment comparison (Table S1). MMP-9 levels in plasma were higher in the ARV initiated than naïve HIV subgroup, possibly reflecting effects of antiretroviral activity.
MMP-2 and -9 were the only MMPs detected in CSF, and were detected in all available samples (n = 9). Because CSF was not acquired from healthy controls, direct group comparisons were precluded. CSF MMP-9 levels in the Chicago Early HIV cohort were generally higher than levels reported for controls in other studies; results reported for CSF MMP-2 levels in controls, however, were widely variable across different studies (Bjerke et al, 2011; Leppert et al, 1998; Niebroj-Dobosz et al, 2010; Yushchenko et al, 2000). Nevertheless, the findings suggest elevated CSF MMP-2 levels in early HIV infection. Evidence from in vivo (Conant et al, 1999; Sporer et al, 1998), autopsy (Johnston et al, 2000), and animal (Johnston et al, 2000) studies indicate elevated brain MMP levels in HIV infection. In this investigation, CSF MMP-2 levels were correlated with loss of white matter integrity (anisotropy) in cerebral white matter and corpus callosum, the largest white matter fiber tract in the brain. CSF MMP-2 levels were also correlated with numerous localized volumetric measurements of basal ganglia, including caudate, putamen, pallidum and accumbens (Table 4). This region is vulnerable to injury in more advanced HIV infection (Becker et al, 2011; Berger and Arendt, 2000).
MMP-2 and MMP-9 are gelatinases that influence blood-brain barrier permeability (Louboutin et al, 2010). In the brain, elevated MMP-2 levels secreted by infected macrophages, microglia, and astrocytes may have neurotoxic effects (Zhang et al, 2003). Elevated MMP-2 and -9 levels have been quantified in brain tissue samples from cerebral cortex and basal ganglia of HIV encephalitis patients and correlate with CD14 mRNA levels (Ghorpade et al, 2001). Correlations with CD14 suggest that elevated MMP levels may be due to increased numbers of immune-activated microglia and perivascular macrophages and to monocyte transmigration.
Interest in circulating factors (i.e. in plasma) follows from evidence implicating systemic monocyte activation in brain injury and cognitive deterioration in HIV infection (Gartner, 2000). The trafficking of blood-borne, activated (CD14+) monocytes to the brain is the presumed vehicle for viral entry. Results from this investigation indicate reduced MMP-2 levels in plasma and likely elevated levels in the brain in HIV infection compared to controls. Other evidence suggests MMP-2 involvement in neurodegeneration. Similar findings of decreased MMP-2 levels in plasma and increased MMP-2 activity in the brain have been reported in Alzheimer’s disease, where MMP-2 may play a role in clearance of amyloid-β plaques (Lim et al, 2011).
MMP-2 levels were nearly an order of magnitude higher in the plasma than in the CSF (n = 9; plasma: 36,462 ± 11,461 pg/mL vs. CSF: 6,700 ± 2,297 pg/mL). Sources of circulating MMP-2 include T-lymphocytes and activated macrophages (Oviedo-Orta et al, 2008). In lymphoid tissue, the ratio of MMP-2 to its inhibitor TIMP-2 is decreased in HIV compared to control subjects (Diaz et al, 2010). Decreased MMP-2 in plasma and in lymphoid tissue may reflect the massive T cell destruction that occurs early in HIV infection in association with unchecked viral expansion. The Millipore antibody used in this study detects both pro- and active forms of MMP-2; therefore, the observed reduction cannot be accounted for by the possibility that MMPs are being cleaved and activated (with the detected domain being degraded). Elevated MMP-2 in CSF may reflect changes occurring in association with increased permeability of the blood-brain barrier in early infection together with constitutively higher levels of MMP-2 in plasma (Louboutin and Strayer, 2012; Xu et al, 2012).
It is important to appreciate that MMPs levels in plasma and CSF, as well as of individual MMPs, are not independent. MMPs participate in a complex protease web, and their effects are determined by factors such as substrate specificity, distribution, and regulatory proteins (Sternlicht and Werb, 2001). MMP-2 and -9 are gelatinases which cleave specific collagen types more effectively than other MMPs. MMP-1 is an interstitial collagenase with preferential affinity toward other collagen types, MMP-7 is a more potent protoglycanase and elastase, and MMP-10 is a stromelysin with specificity for laminin, fibronectin and proteoglycans (Webster and Crowe, 2006). Dysregulated or prolonged MMP activity may pose considerable risk of tissue destruction. Collagen type IV, a substrate of MMP-2 and -9 in the extracellular matrix, is reduced in the brain in HIV infected patients (Buttner et al, 1996). MMPs may also alter biologic activity of cytokines and chemokines (Van Lint and Libert, 2007) and modulate the neurotoxicity of HIV viral proteins (Rumbaugh et al, 2006). It is also important to appreciate that MMPs may also play beneficial roles. MMP-1, for example, is elevated in injured tissues, including the brain (Leake et al, 2000) where it may attenuate HIV neurotoxicity in white matter (Parks et al, 2004; Rumbaugh et al, 2006). In this investigation, plasma MMP-1 levels correlated with anisotropy in cerebral white matter, possibly reflecting this process (Table 5). MMP-7 also correlated with diffusivity throughout the brain and was inversely correlated with viral load, consistent with findings in advanced HIV (Ragin et al, 2011; Ragin et al, 2009). MMPs are involved in regulating neuroinflammation and may figure prominently in aberrant immune activation underlying injury to the brain in HIV infection. In chronic inflammation, all MMPs may be present (Ra and Parks, 2007). In vitro findings indicate MMP changes in response to antiretrovirals (Gramegna et al, 2011; Latronico et al, 2007), suggesting that MMPs may represent potential therapeutic targets. However, evidence of MMP physiologic significance in cognitive function underscores the necessity of further studies before MMPs are targeted for clinical intervention.
This investigation evaluated five different MMPs in plasma and in CSF for patterns of relationship with MR-quantified brain status in an early infection cohort. In particular, MMP-2 displayed patterns of relationship with measurements of multiple brain regions, distinguished stages of the immune response to HIV, and correlated with clinical markers of HIV disease progression. Imaging correlates of plasma MMP-2 levels correspond to regions vulnerable to brain injury in initial stages (Ragin et al, 2012) and later stages of HIV infection. Further prospective studies are necessary to determine the prognostic significance of MMPs. Taken together, these findings support a role of MMP-2 in HIV associated neurological injury in the earliest stages of infection and suggest potential utility as an early plasma marker of neurological vulnerability.
Supplementary Material
Acknowledgements
The authors thank Paul Foryt and Yi Gao.
Funding This work was supported by National Institutes of Health [ABR MH080636].
Footnotes
The authors declare no conflicts of interest.
This information has not been previously presented.
References
- Agrawal SM, Lau L, Yong VW. MMPs in the central nervous system: where the good guys go bad. Seminars in Cell & Developmental Biology. 2008;19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM, Multicenter ACS Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging & Behavior. 2011;5:77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. Journal of Psychopharmacology. 2000;14:214–21. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Bjerke M, Zetterberg H, Edman A, Blennow K, Wallin A, Andreasson U. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. Journal of Alzheimer’s Disease. 2011;27:665–76. doi: 10.3233/JAD-2011-110566. [DOI] [PubMed] [Google Scholar]
- Blair J, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Buttner A, Mehraein P, Weis S. Vascular changes in the cerebral cortex in HIV-1 infection. II. An immunohistochemical and lectinhistochemical investigation. Acta Neuropathologica. 1996;92:35–41. doi: 10.1007/s004010050486. [DOI] [PubMed] [Google Scholar]
- Chen Y, An H, Zhu H, Stone T, Smith JK, Hall C, Bullitt E, Shen D, Lin W. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. NeuroImage. 2009;47:1154–62. doi: 10.1016/j.neuroimage.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi F, Sonnerborg A, Albert J, Gaines H, Norkrans G, Hagberg L, Asjo B, Strannegard O, Fenyo EM. Human immunodeficiency virus infection of the brain. I. Virus isolation and detection of HIV specific antibodies in the cerebrospinal fluid of patients with varying clinical conditions. J Neurol Sci. 1988;85:245–57. doi: 10.1016/0022-510x(88)90184-0. [DOI] [PubMed] [Google Scholar]
- Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–8. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–9. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Diaz A, Garcia F, Mozos A, Caballero M, Leon A, Martinez A, Gil C, Plana M, Gallart T, Gatell JM, Alos L. Lymphoid tissue collagen deposition in HIV-infected patients correlates with the imbalance between matrix metalloproteinases and their inhibitors. Journal of Infectious Diseases. 2010;203:810–3. doi: 10.1093/infdis/jiq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. Ajnr: American Journal of Neuroradiology. 2001;22:277–83. [PMC free article] [PubMed] [Google Scholar]
- Gartner S. HIV infection and dementia. Science. 2000;287:602–4. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- Ghorpade A, Persidskaia R, Suryadevara R, Che M, Liu XJ, Persidsky Y, Gendelman HE. Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia. Journal of Virology. 2001;75:6572–83. doi: 10.1128/JVI.75.14.6572-6583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramegna P, Latronico T, Brana MT, Di Bari G, Mengoni F, Belvisi V, Mascellino MT, Lichtner M, Vullo V, Mastroianni CM, Liuzzi GM. In vitro downregulation of matrix metalloproteinase-9 in rat glial cells by CCR5 antagonist maraviroc: therapeutic implication for HIV brain infection. PLoS ONE [Electronic Resource] 2011;6:e28499. doi: 10.1371/journal.pone.0028499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten R, Sidharthan S, Glielmi C, Du H, Malone F, Ragin A, Edelman R, Wu Y. Reproducibility of automated measurements of Diffusion Tensor Imaging at 3T Using Histogram Analysis. ISMRM. 2011 [Google Scholar]
- Johnston JB, Jiang Y, van Marle G, Mayne MB, Ni W, Holden J, McArthur JC, Power C. Lentivirus infection in the brain induces matrix metalloproteinase expression: role of envelope diversity. Journal of Virology. 2000;74:7211–20. doi: 10.1128/jvi.74.16.7211-7220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating SM, Hanson D, Lebedeva M, Laeyendecker O, Ali-Napo NkL, Owen SM, Stramer SS, Moore RD, Norris PJ, Busch MP. Lower-Sensitivity and Avidity Modifications of the Vitros Anti-HIV 1+2 Assay for Detection of Recent HIV Infections and incidence Estimation. Journal of Clinical Microbiology. 2012;50:3968–3976. doi: 10.1128/JCM.01454-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latronico T, Liuzzi GM, Riccio P, Lichtner M, Mengoni F, D’Agostino C, Vullo V, Mastroianni CM. Antiretroviral therapy inhibits matrix metalloproteinase-9 from blood mononuclear cells of HIV-infected patients. AIDS. 2007;21:677–84. doi: 10.1097/QAD.0b013e328018751d. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature Reviews Neuroscience. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Leake A, Morris CM, Whateley J. Brain matrix metalloproteinase 1 levels are elevated in Alzheimer’s disease. Neuroscience Letters. 2000;291:201–3. doi: 10.1016/s0304-3940(00)01418-x. [DOI] [PubMed] [Google Scholar]
- Leppert D, Ford J, Stabler G, Grygar C, Lienert C, Huber S, Miller KM, Hauser SL, Kappos L. Matrix metalloproteinase-9 (gelatinase B) is selectively elevated in CSF during relapses and stable phases of multiple sclerosis. Brain. 1998;121:2327–34. doi: 10.1093/brain/121.12.2327. [DOI] [PubMed] [Google Scholar]
- Lim NKH, Villemagne VL, Soon CPW, Laughton KM, Rowe CC, McLean CA, Masters CL, Evin G, Li Q-X. Investigation of matrix metalloproteinases, MMP-2 and MMP-9, in plasma reveals a decrease of MMP-2 in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2011;26:779–86. doi: 10.3233/JAD-2011-101974. [DOI] [PubMed] [Google Scholar]
- Liuzzi GM, Mastroianni CM, Santacroce MP, Fanelli M, D’Agostino C, Vullo V, Riccio P. Increased activity of matrix metalloproteinases in the cerebrospinal fluid of patients with HIV-associated neurological diseases. Journal of Neurovirology. 2000;6:156–63. doi: 10.3109/13550280009013159. [DOI] [PubMed] [Google Scholar]
- Louboutin J-P, Agrawal L, Reyes BAS, Van Bockstaele EJ, Strayer DS. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. Journal of Neuropathology & Experimental Neurology. 2010;69:801–16. doi: 10.1097/NEN.0b013e3181e8c96f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louboutin J-P, Strayer DS. Blood-brain barrier abnormalities caused by HIV-1 gp120: mechanistic and therapeutic implications. Thescientificworldjournal. 2012;2012:482575. doi: 10.1100/2012/482575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–55. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–7. [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Annals of Neurology. 1986;19:525–35. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Niebroj-Dobosz I, Janik P, Sokolowska B, Kwiecinski H. Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. European Journal of Neurology. 2010;17:226–31. doi: 10.1111/j.1468-1331.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- Oviedo-Orta E, Bermudez-Fajardo A, Karanam S, Benbow U, Newby AC. Comparison of MMP-2 and MMP-9 secretion from T helper 0, 1 and 2 lymphocytes alone and in coculture with macrophages. Immunology. 2008;124:42–50. doi: 10.1111/j.1365-2567.2007.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nature Reviews Immunology. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biology. 2007;26:587–96. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, Epstein LG. Structural brain alterations can be detected early in HIV infection. Neurology. 2012 doi: 10.1212/WNL.0b013e318278b5b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Ochs R, Du H, Epstein LG, Conant K, McArthur JC. Marked relationship between matrix metalloproteinase 7 and brain atrophy in HIV infection. Journal of Neurovirology. 2011;17:153–8. doi: 10.1007/s13365-011-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin AB, Wu Y, Ochs R, Scheidegger R, Cohen BA, McArthur JC, Epstein LG, Conant K. Serum matrix metalloproteinase levels correlate with brain injury in human immunodeficiency virus infection. J Neurovirol. 2009:1–7. doi: 10.1080/13550280902913271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh J, Turchan-Cholewo J, Galey D, St Hillaire C, Anderson C, Conant K, Nath A. Interaction of HIV Tat and matrix metalloproteinase in HIV neuropathogenesis: a new host defense mechanism. FASEB Journal. 2006;20:1736–8. doi: 10.1096/fj.05-5619fje. [DOI] [PubMed] [Google Scholar]
- Sporer B, Paul R, Koedel U, Grimm R, Wick M, Goebel FD, Pfister HW. Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J Infect Dis. 1998;178:854–7. doi: 10.1086/515342. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Fahey JL, Detels R, Giorgi JV. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to use. Journal of Acquired Immune Deficiency Syndromes. 1989;2:114–24. [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15647–52. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- Webster NL, Crowe SM. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. Journal of Leukocyte Biology. 2006;80:1052–66. doi: 10.1189/jlb.0306152. [DOI] [PubMed] [Google Scholar]
- Wohlschlaeger J, Wenger E, Mehraein P, Weis S. White matter changes in HIV-1 infected brains: a combined gross anatomical and ultrastructural morphometric investigation of the corpus callosum. Clinical Neurology & Neurosurgery. 2009;111:422–9. doi: 10.1016/j.clineuro.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wu Y, Du H, Storey P, Glielmi C, Malone F, Sidharthan S, Ragin A, Tofts PS, Edelman RR. Comprehensive brain analysis with automated high-resolution magnetization transfer measurements. Journal of Magnetic Resonance Imaging. 2012;35:309–17. doi: 10.1002/jmri.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Feng X, Xie X, Zhang J, Wu D, Xu L. HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Research. 2012;1436:13–9. doi: 10.1016/j.brainres.2011.11.052. [DOI] [PubMed] [Google Scholar]
- Yushchenko M, Weber F, Mader M, Scholl U, Maliszewska M, Tumani H, Felgenhauer K, Beuche W. Matrix metalloproteinase-9 (MMP-9) in human cerebrospinal fluid (CSF): elevated levels are primarily related to CSF cell count. Journal of Neuroimmunology. 2000;110:244–51. doi: 10.1016/s0165-5728(00)00339-8. [DOI] [PubMed] [Google Scholar]
- Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark-Lewis I, Overall CM, Power C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–71. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


