Abstract Abstract
Two new monotypic genera, Bergbambos and Oldeania are described for African temperate bamboo species in the tribe Arundinarieae, after a comparison of their morphological characteristics with those of similar species from Asia. Morphological differences are supported by their isolated geographical distributions. Molecular evidence does not support the inclusion of these species in related Asian genera, recognising them instead as distinct lineages. New combinations Bergbambos tessellata and Oldeania alpina are made.
Keywords: Thamnocalamus, Yushania, Arundinarieae, new genus, Africa
Introduction
While Asian temperate bamboos have received critical attention over recent decades (Stapleton 1994, Wong 1995, Li et al. 2006), the generic placement of the temperate bamboos of Africa has not been properly addressed. There seem to be only two temperate bamboo species on the African mainland, currently enumerated most frequently as Thamnocalamus tessellatus (Nees) Soderstrom & R.P. Ellisand Yushania alpina (K. Schum.) W.C. Lin. These species are in tribe Arundinarieae Nees ex Asch. & Graebn., a group also known as the northern temperate clade, identified as a strongly supported monophyletic group from the first molecular analyses of bamboos onwards (Watanabe et al. 1994, Zhang 1996).
Tribe Arundinarieae contains woody bamboos with semelauctant synflorescences (lacking a capability for indeterminate growth from buds subtended by the basal spikelet bracts), ebracteate or partially bracteate synflorescence paraclades (reduced sheathing subtending inflorescence branches) and 3 stamens in each floret. They constitute ca. 800 of the ca. 1400 woody bamboos, and are found in Asia, Africa, and the USA, having a montane or subtropical to temperate distribution.
Molecular studies reviewed by Bamboo Phylogeny Group (2012) suggest that semelauctant inflorescences with 3 stamens and reduced branch sheathing have evolved from tropical bamboos at least twice, once to give the northern temperate clade Arundinarieae of Asia and Africa, spreading to N America, and on separate occasions in Central & South America within the Bambuseae Kunth ex Dumort., principally to give Chusqueinae Bews, with these characters also evolving on a smaller scale within the Arthrostylidiinae Bews and Guaduinae Soderstr. & R. P. Ellis as well.
Most of the older 3-stamened species were placed at some time in Arundinaria Michx., which has 529 combinations, but that genus is now widely recognised as containing only 3 species, all from the Southeast USA (Stapleton et al. 2004, Zeng et al. 2010, Bamboo Phylogeny Group 2012). Treatments of the other species of tribe Arundinarieae vary, according to the breadth of generic concept used, and which characters are given greatest weight. For example, the group of Asian species morphologically closest to Arundinaria could be placed (Zhang et al. 2012) either in a polyphyletic broad interpretation of Arundinaria (e.g. Li et al. 2006), in a polyphyletic broad interpretation of Bashania Keng f. & T.P. Yi (e.g. Keng and Yi 1996), or in the monophyletic Sarocalamus Stapleton (Stapleton et al. 2004, Bamboo Phylogeny Group 2012). The morphologically more distinct species are currently placed in other genera, 27 of which were recognised by Bamboo Phylogeny Group (2012), out of a total of 42 genera that have been described within the tribe.
There appears to have been a rapid and relatively recent diversification within bamboos with 3 stamens, including tribe Arundinarieae (Stapleton et al. 2009, Hodkinson et al. 2010, Zhang et al. 2011, Kelchner and BPG 2013), especially those found in montane and temperate areas such as the Andes, the Himalayas, and Northeast Asia. There have also been several reports of hybridisation, reviewed by Triplett et al. (2010) and Zhang et al. (2012). Hybridisation may well have been common in the bamboos, as mechanisms to avoid it have not been documented. Recent rapid diversification and hybridisation, combined with long generation times, appear to have limited the ability of DNA analyses to resolve phylogenetic patterns and define well supported groups for taxonomy, especially at the generic level (Stapleton et al. 2009, Hodkinson et al. 2010), despite reasonable or sometimes very substantial morphological variation.
In the absence of reliable molecular analyses, for the purpose of descriptive treatments of bamboo species (Li et al. 2006, Wong 1995, Dransfield 2000, Widjaja 1997) a more traditional morpho-geographic approach has been maintained in the classification of Asian bamboos. It has only been possible to use molecular data for the elimination of blatantly polyphyletic groups, rather than the determination of monophyletic ones. Attempts to group the genera substantially (e.g. Clayton and Renvoize 1986, Chao and Renvoize 1989) have resulted in polyphyletic and paraphyletic groups, or clades with weak support that are inconsistent in different analyses.
Rapid recent diversification seems to have spawned a host of small groups, often distinguished by relatively minor characters. Combining them together into a few large genera has not been possible without establishing excessively variable genera that are difficult to define and demonstrably polyphyletic. On the other hand recognising only half of the genera described would still lead to a generic concept that is unusually narrow in the grasses. The latter procedure has been followed (Bamboo Phylogeny Group 2012), largely because it has been found unavoidable if a functional binomial classification system is to be maintained. This is necessary in order to allow pragmatic field identification, and subsequently improve sustainable utilisation and conservation of these species, many of which have a limited range of distribution and are threatened by changes in land use and climate. A substantial proportion of the woody bamboos are yet to be described, and the lack of a functional and stable nomenclatural system for field identification has been a major factor preventing their recognition.
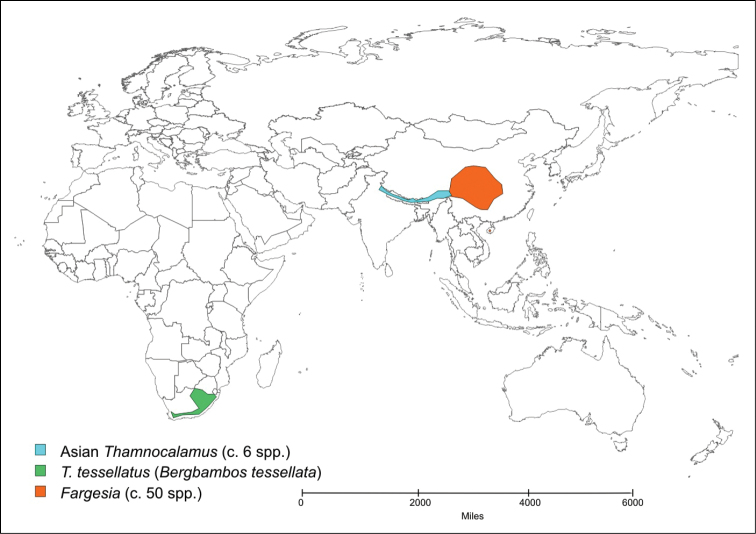
Only two species of temperate bamboo have been described from the African mainland. Thamnocalamus tessellatus (Nees) Soderstrom & R.P. Ellis is from mountains in southern Africa, while Yushania alpina (K. Schum.) W.C. Lin is from mountains in several countries across tropical Africa. Yushania alpina was described initially in Arundinaria, and Thamnocalamus tessellatus was soon transferred into that genus from Nastus Juss. They were more recently moved into the morphologically closer Asian genera, Thamnocalamus Munroand Yushania Keng f., the geographically closest representatives of which are found in the Western Himalayas, Map 1 and Map 2.
Map 1.
Distribution of Thamnocalamus tessellatus, Thamnocalamus in Asia, and Fargesia.
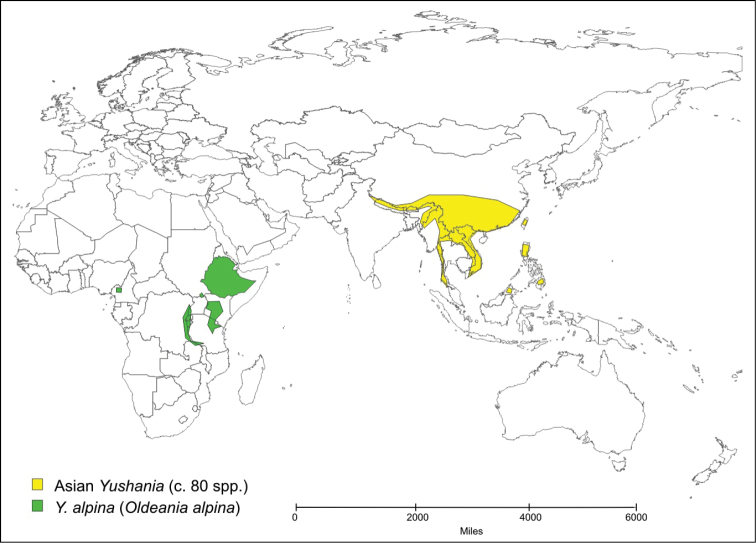
Map 2.
Distribution of Yushania alpina and Asian Yushania species.
Three further, less well known species, Thamnocalamus ibityensis (A. Camus) Ohrnb., Yushania madagascariensis (A. Camus) Ohrnb. and Yushania humbertii (A. Camus) Ohrnb. (including Yushania ambositrensis (A. Camus) Ohrnb.) were described from Madagascar. Thamnocalamus ibityensis has been considered conspecific with Thamnocalamus tessellatus (Chao & Renvoize, 1989), but it would appear to have substantially different branch sheathing. The two Yushania species would appear to share characteristics with Yushania alpina, but their culms, branching and culm sheaths are not known. Yushania ambositrensis resolved in a clade with Yushania alpina (Triplett, 2008), but it is not clear how closely related they really are to Yushania alpina, or to each other, and which species names should be recognised. Further field work on temperate species of Madagascar is required, as existing collections are incomplete, although any such species may have already become extinct.
Comparison of morphological characters
Systematics within the grass family has traditionally given greater weight to floral than to vegetative characters. This has often led to polyphyletic genera in the bamboos, the superficiality of their similarities and their separate origins only being revealed by in-depth morphological investigations and/or molecular studies. In order to allow deeper, more objective morphological comparisons and to allow inclusion of consistent and accurate vegetative as well as floral characters in descriptions, the morphology of woody bamboos has been reviewed in depth (Stapleton 1997, available online). Recent bamboo treatments (Judziewicz et al. 1999, Li et al. 2006, Triplett et al. 2006, BPG 2012) have employed these revised concepts and terminologies, and they are followed here.
The characters and character states considered important at the generic level for distinguishing the two African species from similar Asian genera are given in Table 1.
Table 1.
Principal morphological characters of Bergbambos, Oldeania, and Asian members of 5 similar genera.
|
Bergbambos (Thamnocalamus tessellatus) |
Thamnocalamus | Fargesia |
Oldeania (Yushania alpina) |
Yushania | Borinda | Chimonocalamus | |
|---|---|---|---|---|---|---|---|
| synflorescence | raceme, not unilateral | raceme to panicle, not unilateral | raceme, unilateral | panicle, not unilateral | panicle, not unilateral | panicle, not unilateral | panicle, not unilateral |
| paraclades | largely ebracteate | substantially bracteate | variably bracteate | largely ebracteate | largely ebracteate | largely ebracteate | largely ebracteate |
| pedicel | scabrous | glabrous | glabrous | glabrous | glabrous | glabrous | glabrous |
| fertile florets | 1 | 2–several | 2–several | 2–several | 2–several | 2–several | 2–several |
| glume bud remnants | absent | variable | present | absent | variable | variable | absent |
| rhizomes | short-necked | short-necked | short-necked | long-necked | long-necked | short-necked | short-necked |
| clump form | unicaespitose | unicaespitose | unicaespitose | culms solitary | pluricaespitose | unicaespitose | unicaespitose |
| nodes | without roots | without roots | without roots | with short roots | without roots | without roots | with root thorns |
| supranodal ridge | obscure | obscure | obscure | well developed | obscure | obscure | well developed |
| culm internodes | terete | terete | terete | sulcate | terete | terete | terete |
| branch sheathing | reduced | complete | reduced | reduced | reduced | reduced | complete |
| branch orientation | erect | erect | erect | spreading | erect to spreading | erect | spreading |
| mid-culm branches | 5–7 | 3–8 | 5–7 | 3–7 | 5–11 | 5–7 | 3 |
| culm sheath blades | erect or reflexed | usually erect | erect or reflexed | usually reflexed | erect or reflexed | erect or reflexed | usually reflexed |
Thamnocalamus tessellatus
Previous generic placements of Thamnocalamus tessellatus were based upon an incomplete knowledge of its morphology. Nastus tessellatus Nees was described before its flowers were known, and transferred into Arundinaria (Munro 1868) simply as it bore “very great resemblance” to that genus. Later discovery of its flowers has shown that it indeed has 3 stamens, rather than the 6 of Nastus, but it has pachymorph rhizomes (see Stapleton 1997: fig. 1) rather than the leptomorph rhizomes of Arundinaria.
It was transferred into Thamnocalamus largely on the basis of leaf anatomical characters by Soderstrom and Ellis (1982), who found that Arundinaria tessellata shared 10 characters out of 11 with Thamnocalamus spathiflorus (Trin) Munro, while it only shared 7 characters with Fargesia nitida (Mitford) Keng f. However, Arundinaria tessellata also shared only 5 characters with Thamnocalamus aristatus E.G. Camus, while the possibly conspecific Thamnocalamus spathiflorus and Thamnocalamus aristatus themselves only shared 6 out of 11 characters. When morphological characters other than those of leaf anatomy, along with more recent molecular results are taken into account, it would appear that the anatomical characters used by Soderstrom and Ellis (1982) are more informative at the level of species or below rather than at generic level.
The synflorescence of Thamnocalamus tessellatus has been well illustrated in Hooker’s Icones Plantarum (Prain 1913: Tab 2930 http://www.botanicus.org/page/1349516), and by Soderstrom and Ellis (1982). When examined closely, it can be seen that the synflorescence of Thamnocalamus tessellatus has similarities to those of both Thamnocalamus and Fargesia Franchet, see Table 1, as they are compressed, and are associated with several supporting sheaths. However, while Thamnocalamus has loose racemose panicles, Thamnocalamus tessellatus, like Fargesia, consistently bears short racemes. These are structurally very similar to those of Fargesia, but differences arise in the arrangement of the florets. In Fargesia the racemes are held tightly within imbricating sheaths, which can extend well beyond the spikelets. Development within the sheaths forces them to emerge to one side and appear unilateral, with the pedicels tightly pressed against the rhachis. Those of Thamnocalamus tessellatus are more cylindrical, the spikelets not so constricted by the sheaths, and the pedicels are free to develop in a normal distichous fashion, Figure 1.
Figure 1.
Raceme of Thamnocalamus tessellatus (A), compared to: B Fargesia nitida, lateral view with enclosing sheaths; C Fargesia nitida with enclosing sheaths removed D Fargesia nitida, dorsal view, sheaths removed. A from Soderstrom and Ellis (1982), drawn by A. R. Tangerini, © Smithsonian Institution. B, C, D from Stapleton 1061b. Scale bars 2 mm.
In addition, in Thamnocalamus tessellatus the pedicels are scabrous, the glumes of each spikelet are basally tight and contain no vestigial bud remnants, and the racemes are usually largely ebracteate. The usually single fertile florets also distinguish Thamnocalamus tessellatus from other Thamnocalamus and Fargesia species, but this character should be treated with caution as it can be a specific as well as a generic character.
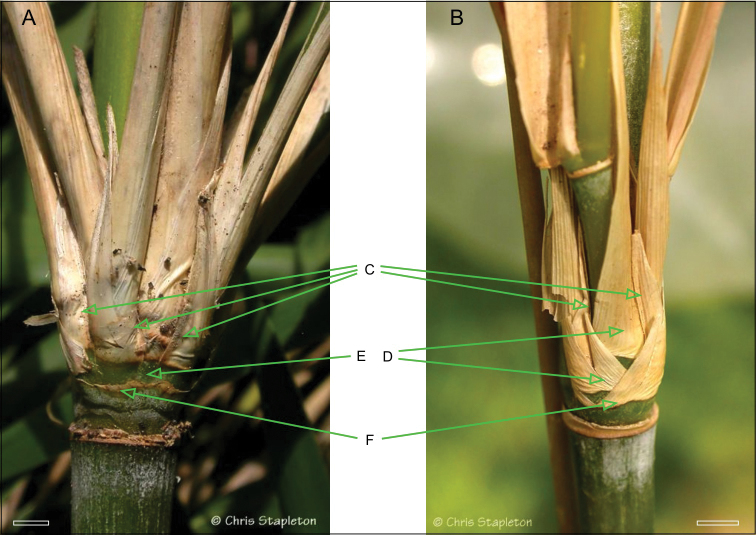
Thamnocalamus tessellatus also hasvegetative characteristics that distinguish it, notably from Asian members of Thamnocalamus, (see Table 1). A close inspection of the branching reveals not the pattern seen in species such as Thamnocalamus crassinodus (T.P. Yi) Demoly, but instead the substantial reduction in sheathing seen in Fargesia, Yushania, and Borinda Stapleton, Figure 2.
Figure 2.
Comparison of branch complement sheathing from mid-culm nodes of Thamnocalamus tessellatus (A) and Thamnocalamus crassinodus (B) C Lateral branch prophylls D Sheaths obscuring prophyll bases in Thamnocalamus crassinodus E Equivalent sheaths completely absent in Thamnocalamus tessellatus, prophylls visible F Branch bud prophyll, removed in A, still present in B. Scale bars 1 cm. From http://www.bamboo-identification.co.uk
The branches of Thamnocalamus tessellatus are subequal, arranged side by side through strong compression of the basal internodes of the central branch, accompanied by loss of some of the sheaths at the nodes, Fig. 2A, cf Thamnocalamus crassinodus, Fig. 2B. This allows lateral branch prophylls to be seen side by side without any intervening sheaths. These patterns were contrasted by Stapleton (1991; 1994: fig. 1; 1997: fig. 2), and also illustrated for Thamnocalamus tessellatus by Soderstrom and Ellis (1982: fig.1, fig. 4).
In addition to the synflorescence and branching, Thamnocalamus tessellatus also differs in minor details that are harder to quantify, including the more varied orientation of the foliage leaves, and the delicate appearance of its oral setae and their more varied orientation, Figure 3.
Figure 3.
Thamnocalamus tessellatus. A Random orientation of leaf blade; B and C Irregular orientation of delicate oral setae. Scale bars A 10 cm, B and C 2 mm. From http://www.bamboo-identification.co.uk/html/tessellatus.html
Thus in terms of vegetative macro-morphological characteristics important at the generic level, Thamnocalamus tessellatus is closer to Fargesia than to Thamnocalamus, but can be distinguished from both. In general appearance it resembles a coastal species of Pleioblastus Nakai from Japan, with rather loose clumps, erect culms with short branches bearing coarse, irregularly arranged foliage with persistent sheaths. This contrasts with the delicate foliage leaves, all oriented towards the light on pendulous branches seen in Himalayan speciesof Thamnocalamus and in Fargesia. This is likely to be associated with the more open ecological habitat in which Thamnocalamus tessellatus is found, rather than the darker forest understorey habitats of Asian Thamnocalamus and Fargesia species.
Yushania alpina
The synflorescence of Yushania alpina is practically indistinguishable from those of several Asian and American bamboos, including species of Arundinaria, Sarocalamus, and Yushania—an open panicle with nearly complete reduction in sheathing at points of branching so that it is essentially ebracteate. However, the sheaths are often reduced to small tough bracts, as well as the more delicate sheath remnants or tufts of hairs seen in similar genera. In addition the lateral spikelets are more often sessile or subsessile, without a long pedicel. However, these characters are relatively minor and quite variable.
Yushania alpina is more distinct vegetatively. Reaching heights of up to 20m in its natural habitat, the tall, very erect culms are potentially much larger than those of any Asian species of Yushania, which only reach a maximum height of about 7m. Culm nodes and branching also differ substantially from those of Asian species of Yushania, Figure 4.
Figure 4.
Yushania alpina. A culm sheath B leaf sheaths, culm node with ring of thorn-like aerial roots and distinct supra-nodal ridge, sulcate internode, and dominant central branch. Scale bars top right, 2 cm.
Branches vary in size more than those of Asian Yushania species. The central branch is strongly dominant, and the first two lateral branches are also strong. The orientation of the branches is less erect than those of most species of Yushania, becoming nearly horizontal. Above the branches the internode is distinctly sulcate, much more prominently than is seen in Asian Yushania species, as a result of the development of strong branches. Moreover there is often a dense ring of short, partially developed aerial roots at nodes in the lower part of the culm, often extending into the mid-culm region as well. This character is only known in species of Chimonocalamus Hsueh & T.P. Yi, and the leptomorph-rhizomed Chimonobambusa Makino among the Asian temperate bamboos. The roots are not as sharp and thorn-like as those seen in Chimonocalamus and Chimonobambusa, but they can be very distinct and prominent. Nodes have a distinct infranode between the culm sheath attachment and the supranodal ridge, which is well developed, Figure 5.
Figure 5.
Yushania alpina A tall culms arising separately with prominent supra-nodal ridges (arrowed), Rwenzori, Uganda B culm node with ring of thorn-like aerial roots (arrowed), Mt. Kenya.Scale bars A 25 cm, B 5 cm. Photos courtesy of: (A) Peter Gill, (B) Harry Jans, www.jansalpines.com
In its natural habitat, the open stands have a widely spaced appearance closer to that of a species of Phyllostachys Siebold & Zucc., rather than the denser thickets of Asian Yushania species, because the rhizomes have consistently long necks, giving solitary culms rather than the denser clusters of pluricaespitose culms seen in Asian species of Yushania.
Branch structure and sheathing is difficult to distinguish from that of Yushania or Fargesia. Although the prophyll is usually 2-keeled, there is replication side by side of lateral branch initials without intervening sheaths. In this way it differs fundamentally from Chimonocalamus, which has only 3 branches and full sheathing.
Discussion
The morphological differences between Thamnocalamus tessellatus, Yushania alpina and other representatives of these and similar Asian genera suggest that although the two African bamboos share several characters and presumably common ancestors with Asian bamboos, they are not as closely related to their Asian relatives as previously thought.
The morphological distinctions are supported by geographical isolation, (see Maps 1 and 2). Long-distance dispersal of temperate bamboos is highly unlikely because of a lack of any specialized seed dispersal mechanism or dormancy, brief viability of seed, exacting habitat requirements, and extremely infrequent flowering (Stapleton et al. 2004).
Together the morphological distinctions and geographical isolation justify the recognition of two new genera, following the existing relatively narrow generic concepts applied in the northern temperate clade, tribe Arundinarieae.
The new genera are keyed out below along with their 6 morphologically closest relatives in the tribe including the two Asian genera with distinct nodal thorns, as well as the North American type genus of the tribe, Arundinaria, and its Asian analogue, Sarocalamus.
Key to Bergbambos, Oldeania and related genera
| 1 | Rhizome leptomorph | 2 |
| – | Rhizome pachymorph | 4 |
| 2 | Basal culm nodes with thorns, branches spreading | Chimonobambusa |
| – | Basal culm nodes without thorns, branches erect | 3 |
| 3 | Pedicels glabrous, leaf blades thick, SE USA | Arundinaria |
| – | Pedicels not glabrous, leaf blades thin, Himalayas & W China | Sarocalamus |
| 4 | Branches 3, all sheaths developed, basal nodes with thorns | Chimonocalamus |
| – | Branches 3-15, sheathing reduced, basal culm nodes with or without thorns | 5 |
| 5 | Rhizomes long or variable in length, clumps open or spreading | 6 |
| – | Rhizomes consistently short, culms in single clumps | 7 |
| 6 | Nodes raised, basal culm nodes usually with thorns, Africa | Oldeania |
| – | Nodes not raised, basal culm nodes without thorns, Asia | Yushania |
| 7 | Branch sheathing complete | Thamnocalamus |
| – | Branch sheathing reduced | 8 |
| 8 | Synflorescence branching paniculate | Borinda |
| – | Synflorescence branching racemose | 9 |
| 9 | Racemes unilateral, W China | Fargesia |
| – | Racemes not unilateral, Africa | Bergbambos |
Sufficient data is now available to test whether this classification would gain support from molecular phylogenetic evidence. These two African species were not clearly resolved with Asian representatives of any genera in any molecular studies. For example, in a comparison of ITS sequences (Guo et al. 2002), Thamnocalamus tessellatus did not resolve with the type species of Thamnocalamus, Thamnocalamus spathiflorus, and its position varied between topologies. In the nuclear ribosomal ITS analysis of Hodkinson et al. (2010), Yushania alpina did not group with other Yushania species or closely with any other taxon. Weak associations between Yushania alpina and Chimonocalamus species were found by Guo and Li (2004) and Triplett (2008), which is interesting as they share possession of aerial roots developed into thorn-like structures, although they differ in other ways. However, neither Yushania alpina nor Thamnocalamus tessellatus resolved with putative relatives in these or similar genera of temperate bamboos in the most comprehensive studies undertaken so far, using sequences from 8 regions of cpDNA in 146 species and 26 genera (Zeng et al. 2010), and 108 bamboos from 25 genera using plastid DNA and nuclear GBSSI gene sequences (Zhang et al. 2012).
The molecular data would suggest that their inclusion in Asian genera would render those genera polyphyletic. Because their monotypic status is considered likely they could not be supported as monophyletic groups themselves in a classification based solely on molecular phylogeny. However, Zeng et al. (2010) and Zhang et al. (2012) considered them both to represent distinct lineages, and it is not possible to place them in well supported meaningful monophyletic groups except the tribe Arundinarieae. Therefore while the molecular data would not allow the diagnosis of monophyletic genera for the African bamboos following a strict Hennigian cladistic analysis, neither their current placement in Thamnocalamus and Yushania, nor placement in any other existing genus receives any support either. Continuing to include these bamboos in Asian genera causes serious problems when describing or distinguishing between those genera.
Although woody bamboos are considered to have evolved originally in Gondwanaland rather than Eastern Asia (Hodkinson et al. 2010), these African representatives are nested within the northern temperate clade, the tribe Arundinarieae, with a largely Asian distribution. This is estimated to have diverged from other woody bamboos around 23 mya (Hodkinson et al. 2010), 29 mya (Bouchenak-Khelladi et al. 2010) or 37.5 mya (Christin et al. 2008), but to have radiated only ca. 9 mya (Bouchenak-Khelladi et al. 2010) 10 mya (Hodkinson et al. 2010), or 19 mya (Christin et al. 2008). Peng et al. (2013) after sequencing 95% of the Phyllostachys edulis genome found evidence of whole genome duplication 7–12 mya, supporting the more recent dates.
Collision of tectonic plates has been suggested as a likely cause of this rapid radiation (Stapleton et al. 2009, Hodkinson et al. 2010). African and Indian plates met the Eurasian plate around that time, allowing a biotic interchange and subsequent radiation and diversification of Gondwanan elements into a wealth of new habitats. However, the temperate ancestors of these two African bamboo genera seem to have diverged around the same time that temperate bamboos arrived in Eastern Asia. Inclusion of endemic temperate bamboos from S India, Sri Lanka and Madagascar in a molecular phylogeny is required before any conclusions can be drawn as to where bamboos from the northern temperate clade first evolved, but there seems no evidence for an African origin, and it seems more likely that temperate bamboos radiated from India to Asia, Africa, and N America.
Nomenclature
Bergbambos
Stapleton gen. nov.
urn:lsid:ipni.org:names:77131102-1
http://species-id.net/wiki/Bergbambos
Remarks.
Differing from Arundinaria and Sarocalamus and similar to Thamnocalamus and Fargesia in its short-necked pachymorph rather than leptomorph rhizomes, and its compressed synflorescences. Differing from Borinda and Thamnocalamus in its racemose rather than paniculate synflorescence branching. Differing from Fargesia in the distichous rather than unilateral arrangement of spikelets in the racemes, the spikelets usually having only one fertile floret, and the scabrous pedicels. Differing from Thamnocalamus in the branch complement with reduced sheathing, and from Fargesia in the more varied orientation of the leaf blades.
Type.
Bergbambos tessellata (Nees) Stapleton comb. nov. urn:lsid:ipni.org:names:77131104-1 Basionym: Nastus tessellatus Nees, Fl. Afr. Austr. 1: 463. 1841. Arundinaria tessellata (Nees) Munro; Thamnocalamus tessellatus (Nees) Soderstrom & R.P. Ellis. Type: S Africa, Katberg, 4000–5000ft, J.F. Drège s.n. (lectotype, designated in Soderstrom & Ellis 1982, pg. 54: K!, http://apps.kew.org/herbcat/getImage.do?imageBarcode=K000345516
Rhizome pachymorph, short-necked, giving dense clumps. Culms to 7 m tall, diam. to 2 cm, nodding to pendulous, terete, smooth, nodes not raised and unarmed. Mid-culm branch complement initially with 5–7 main branches, erect, sheathing reduced. Culm sheaths persistent, tough. Leaf sheaths several to many, persistent, blades thick with random orientation. Synflorescence semelauctant, racemose, branch sheathing occasionally a soft sheath remnant, usually absent. Racemes not unilateral. Spikelets shortly pedicellate with 1(–2) fertile florets, pedicel scabrous. Empty glumes 2, no bud remnants. Lemma and palea similar in length. Stamens 3, filaments free. Stigmas 3. Lodicules 3.
Name Bergbambos from the Afrikaans name (Bergbamboes) in South Africa.
This genus would appear to be monotypic, confined to the mountains of South Africa, Lesotho and Swaziland.
Oldeania
Stapleton gen. nov.
urn:lsid:ipni.org:names:77131103-1
http://species-id.net/wiki/Oldeania
Remarks.
Differing from Arundinaria and Sarocalamus and similar to Yushania in its long-necked pachymorph rather than leptomorph rhizomes, though similar to all in its open panicles. Differing from Yushania in its sulcate culm internodes, fewer, more horizontal branches, culm nodes with well developed supra-nodal ridge and often thorn-like aerial roots. Similar to Chimonocalamus in its panicles and thorn-like roots at culm nodes, but differing in its multiple branches with reduced sheathing and sulcate culm internodes.
Type.
Oldeania alpina (K. Schum.) Stapleton comb. nov. urn:lsid:ipni.org:names:77131105-1 Basionym Arundinaria alpina K. Schum. in Engler, Pflanzenwelt Ost-Afrikas 5: 117. 1895. Sinarundinaria alpina (K. Schum.) C.S. Chao & Renvoize; Yushania alpina (K. Schum.) W.C. Lin. Type: Kenya, Kikiju, G.A. Fischer 672 (holotype: B n.v., destroyed).
Rhizome pachymorph, long-necked, giving open stands and solitary culms. Culms to 15(–20) m tall, diam. to 6(–10) cm, erect to nodding, terete with shallow sulcus above branches, smooth, nodes with prominent supranodal ridge, in lower to mid culm a nodal ring of dense, short, hard, and thorn-like aerial roots often well developed. Mid-culm branch complement initially with 3–5 main branches, spreading, sheathing reduced. Culm sheaths deciduous, tough. Leaf sheaths several to very many, blades thick. Synflorescence semelauctant, paniculate, branch sheathing reduced to hard bracts, soft sheath remnants or hairs. Spikelets pedicellate with several fertile florets, pedicel scabrous. Empty glumes 2, bud remnants present or absent, fertile glumes 4–8. Lemma and palea similar in length. Stamens 3, filaments free. Stigmas 2. Lodicules 3.
Name Oldeania from the Maasai common name (Oldeani) in Tanzania.
Currently only the type species can be reliably placed in the genus, which thus has a distribution across tropical Africa from Cameroon in the west to E Africa, where it occurs from Ethiopia south to Tanzania. There is a possibility that species from Madagascar will be placed in this genus when they are better known, but they may be conspecific or even introduced. It provides important montane wildlife habitats and food, notably for the critically endangered Mountain Gorilla, Gorilla beringei beringei.
The holotype, G.A. Fischer 672, was destroyed by fire during the 1939–1945 World War. No trace of the type collection or any duplicate has been found in surviving components of the Berlin collections, nor in other herbaria, including the Hamburg collections taken to Russia and recently repatriated (Poppendieck pers. comm.). The likelihood of substantial infraspecific variation, the possibility of further species, and the lack of other collections from the type locality together make it inadvisable to select a neotype or epitype until new collections have been made.
Supplementary Material
Acknowledgements
The American Bamboo Society is thanked for providing open access publication fees for this paper. Dr Hans-Helmut Poppendieck, Curator of Phanerogams at Herbarium Hamburgense is thanked for information relating to the missing type of Arundinaria alpina. Dr Ximena Londoño kindly looked for ms names while at the Smithsonian Institute, and Dr John Grimshaw provided information on African local names. Dr Sylvia Phillips arranged for collection of DNA samples in Ethiopia. Dr Eric Boa, Dr Peter Gill, and Harry Jans provided photos from Ethiopia and Uganda. Dr Martin Cheek at RBG Kew encouraged me to persevere with African bamboos. Journal editor Dr Leonardo Versieux and the anonymous reviewers are thanked for their valuable comments.
References
- Bamboo Phylogeny Group. (2012) An updated tribal and subtribal classification for the Bambusoideae (Poaceae). In: Gielis J, Potters G. (Eds) Proceedings of the 9th World Bamboo Congress, 10-12 April 2012, Antwerp, Belgium, 3–27 [Google Scholar]
- Bouchenak-Khelladi Y, Verboom GA, Savolainen V, Hodkinson TR. (2010) Biogeography of the grasses (Poaceae): a phylogenetic approach to reveal evolutionary history in geographical space and geological time. Botanical Journal of the Linnean Society 162: 543-557.10.1111/j.1095-8339.2010.01041.x [Google Scholar]
- Chao CS, Renvoize SA. (1989) A revision of the species described under Arundinaria (Gramineae) in Southeast Asia and Africa. Kew Bulletin 44(2): 349-367.10.2307/4110809 [Google Scholar]
- Christin PA, Besnard G, Samaritani E, Duvall MR, Hodkinson TR, Savolainen V, Salamin N. (2008) Oligocene CO2 decline promoted C4 photosynthesis in grasses. Current Biology 18: 1-7.10.1016/j.cub.2007.11.058 [DOI] [PubMed] [Google Scholar]
- Clayton WD, Renvoize SA. (1986) Genera Graminum: Grasses of the World. Royal Botanic Gardens, Kew [Google Scholar]
- Dransfield S. (2000) Woody Bamboos (Gramineae–Bambusoideae) of Madagascar. In: Jacobs SWL, Everett J. (Eds) Grasses: Systematics & Evolution, CSIRO, 43–50 [Google Scholar]
- Guo ZH, Chen YY, Li DZ. (2002) Phylogenetic studies on the Thamnocalamus group and its allies (Gramineae: Bambusoideae) based on ITS sequence data. Molecular Phylogenetics and Evolution 22: 20–30 [online 5 DEC 2001]10.1006/mpev.2001.1039 [DOI] [PubMed] [Google Scholar]
- Guo ZH, Li DZ. (2004) Phylogenetics of the Thamnocalamus group and its allies (Gramineae: Bambusoideae): inference from the sequences of GBSSI gene and ITS spacer. Molecular Phylogenetics and Evolution 30: 1–12 http://ir.kib.ac.cn:8080/bitstream/151853/11245/1/201109070015.PDF10.1016/S1055-7903(03)00161-1 [DOI] [PubMed] [Google Scholar]
- Hodkinson TR, Ní Chonghaile G, Sungkaew S, Chase MW, Salamin N, Stapleton CMA. (2010) Phylogenetic analyses of plastid and nuclear DNA sequences indicate a rapid late Miocene radiation of the temperate bamboo tribe Arundinarieae (Poaceae, Bambusoideae). Plant Ecology & Diversity 3(2): 109-120.10.1080/17550874.2010.521524 [Google Scholar]
- Judziewicz EJ, Clark LG, Londoño X, Stern MJ. (1999) American Bamboos: Natural History of the Native New World Bamboos. Smithsonian Institution Press, Washington [Google Scholar]
- Kelchner SA, Bamboo Phylogeny Group. (2013) Higher level phylogenetic relationships within the bamboos (Poaceae: Bambusoideae) based on five plastid markers. Molecular Phylogenetics and Evolution 67(2): 404-413.10.1016/j.ympev.2013.02.005 [DOI] [PubMed] [Google Scholar]
- Keng PC, Yi TP. (1996) Bashania. In: Keng PC, Wang ZP. (Eds) Gramineae (Poaceae), Bambusoideae, Flora Reipublicae Popularis Sinicae 9(1), Science Press, Beijing, 612–620 [Google Scholar]
- Li DZ, Wang ZP, Zhu ZD, Xia NH, Jia LZ, Guo ZH, Yang GY, Stapleton CMA. (2006) In: Wu ZY, Raven PH, Hong DY. (Eds) Bambuseae, Flora of China 22 Poaceae. Science Press, Beijing & Missouri Botanical Garden Press, St. Louis, 7–180 http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=20753 [Google Scholar]
- Munro W. (1868) A monograph of the Bambusaceae. Transactions of the Linnean Society of London 26: 1-157 [Google Scholar]
- Peng ZH, Lu Y, Lubin Li, Zhao Q, Feng Q, Gao ZM, Lu HY, Hu T, Yao N, Liu KY, Li Y, Fan DL, Guo YL, Li WJ, Lu YQ, Weng QJ, Zhou CC, Zhang L, Huang T, Zhao Y, Zhu CR, Liu XG, Yang XW, Wang T, Miao K, Zhuang CY, Cao XL, Tang WL, Liu GS, Liu YL, Chen J, Liu ZJ, Yuan LC, Liu ZH, Huang XH, Lu TT, Fei BH, Ning ZM, Han B, Jiang ZH. (2013) The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nature Genetics 45: 456–461 http://www.nature.com/doifinder/10.1038/ng.2569 [DOI] [PubMed] [Google Scholar]
- Prain D. (1913) Hooker’s Icones Plantarum, Fourth Series 10. Dulau, London, UK: http://www.botanicus.org/page/1349516 [Google Scholar]
- Soderstrom TR, Ellis RP. (1982) Taxonomic status of the endemic South African bamboo, Thamnocalamus tessellatus. Bothalia 14: 53-67 [Google Scholar]
- Stapleton CMA. (1991) A morphological investigation of some Himalayan bamboos with an enumeration of taxa in Nepal and Bhutan. PhD thesis. University of Aberdeen, UK [Google Scholar]
- Stapleton CMA. (1994) The bamboos of Nepal and Bhutan Part II: Arundinaria, Thamnocalamus, Borinda, and Yushania (Gramineae: Poaceae, Bambusoideae). Edinburgh Journal of Botany 51(2): 275–295 http://www.bamboo-identification.co.uk/EJB2_fig.pdf [Google Scholar]
- Stapleton CMA. (1997) The morphology of woody bamboos. In: Chapman GP. (Ed) The Bamboos.Linnean Society of London Symposium Series, Academic Press, London, 251–267 http://www.bamboo-identification.co.uk/MORPH8_compr_encr.pdf [Google Scholar]
- Stapleton CMA, Ní Chonghaile G, Hodkinson TR. (2004) Sarocalamus, a new Sino-Himalayan bamboo genus (Poaceae–Bambusoideae). Novon 14: 345–349 http://biodiversitylibrary.org/page/644003 [Google Scholar]
- Stapleton CMA, Hodkinson TR, Ní Chonghaile G. (2009) Molecular phylogeny of Asian woody bamboos: Review for the Flora of China. Bamboo Science and Culture, J. of the American Bamboo Society 22(1): 5–25 http://www.bamboo-identification.co.uk/MPAWB_open.pdf [Google Scholar]
- Triplett JK, Weakley AS, Clark LG. (2006) Hill cane (Arundinaria appalachiana), a new species of bamboo (Poaceae: Bambusoideae) from the southern Appalachian Mountains. Sida 22: 79–85 [Google Scholar]
- Triplett JK. (2008) Phylogenetic relationships among the temperate bamboos (Poaceae: Bambusoideae) with an emphasis on Arundinaria and allies. PhD thesis. Iowa State University, US [Google Scholar]
- Triplett JK, Oltrogge KA, Clark LG. (2010) Phylogenetic relationships and natural hybridization among the North American woody bamboos (Poaceae: Bambusoideae: Arundinaria). American Journal of Botany 97(3): 471-492 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Ito M, Kurita S. (1994) Chloroplast DNA phylogeny of Asian bamboos (Bambusoideae, Poaceae) and its Systematic Implication. Journal of Plant Research 107: 253-261 [Google Scholar]
- Widjaja EA. (1997) New Taxa in Indonesian Bamboos. Reinwardtia 11: 57-152 [Google Scholar]
- Wong KM. (1995) The Bamboos of Peninsular Malaya. Malayan Forest Records No. 41. Forest Research Institute, Malaysia, Kuala Lumpur [Google Scholar]
- Zeng CX, Zhang YX, Triplett JK, Yang JB, Li DZ. (2010) Large multi-locus plastid phylogeny of the tribe Arundinarieae (Poaceae: Bambusoideae) reveals ten major lineages and low rate of molecular divergence. Molecular Phylogenetics & Evolution 56: 821–839 http://ir.kib.ac.cn:8080/bitstream/151853/3020/1/201112200016.pdf10.1016/j.ympev.2010.03.041 [DOI] [PubMed] [Google Scholar]
- Zhang WP. (1996) Phylogeny and classification of the bamboos (Poaceae: Bambusoideae) based on molecular and morphological data. PhD dissertation. Iowa State University
- Zhang YJ, Ma PF, Li DZ. (2011) High-throughput sequencing of 6 bamboo chloroplast genomes: phylogenetic implications for temperate woody bamboos (Poaceae: Bambusoideae). PLoS ONE 6(5): e20596.10.1371/journal.pone.0020596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YX, Zeng CX, Li DZ. (2012) Complex evolution in Arundinarieae (Poaceae: Bambusoideae): Incongruence between plastid and nuclear GBSSI gene phylogenies. Molecular Phylogenetics and Evolution 63: 777–797 http://ir.kib.ac.cn:8080/bitstream/151853/15057/1/Zhang-2012-Complex evolution in.pdf10.1016/j.ympev.2012.02.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.