Abstract
Steroid hormones are well-recognized suppressors of the inflammatory response, however, their cell- and tissue-specific effects in the regulation of inflammation are far less understood, particularly for the sex-related steroids. To determine the contribution of progesterone in the endothelium, we have characterized and validated an in vitro culture system in which human umbilical vein endothelial cells constitutively express human progesterone receptor (PR). Using next generation RNA-sequencing, we identified a selective group of cytokines that are suppressed by progesterone both under physiological conditions and during pathological activation by lipopolysaccharide. In particular, IL-6, IL-8, CXCL2/3, and CXCL1 were found to be direct targets of PR, as determined by ChIP-sequencing. Regulation of these cytokines by progesterone was also confirmed by bead-based multiplex cytokine assays and quantitative PCR. These findings provide a novel role for PR in the direct regulation of cytokine levels secreted by the endothelium. They also suggest that progesterone-PR signaling in the endothelium directly impacts leukocyte trafficking in PR-expressing tissues.
Keywords: Steroid hormone, Immune cell, Reproduction, Inflammation
1. Introduction
Inflammation contributes to the susceptibility and progression of many diseases that exhibit gender based differences in prevalence. These include, but are not limited to, autoimmune disease, cardiovascular disease and sexually transmitted infections (Kaushic et al., 2011, McCombe et al., 2009 and Meyer et al., 2006). The prevailing hypothesis is that endocrine–immune interactions drive this sexual dimorphism by affecting the sensitivity to various inflammatory stimuli. Evidence for this emanates from studies demonstrating the requirement for the immune system in hormonally controlled processes including implantation, cycling, and pregnancy (Challis et al., 2009, Gilliver, 2010, Jones, 2004, King and Critchley, 2010, Red-Horse and Drake, 2004 and van Mourik et al., 2009). For example, symptoms of rheumatoid arthritis and multiple sclerosis are reduced during pregnancy, suggesting that hormones not only modulate local inflammatory reactions, but also can affect systemic immune responses as well (Adams Waldorf and Nelson, 2008, Hughes, 2012 and Martocchia et al., 2011). While much is known of the cellular and molecular control of the immune system by estrogen, glucocorticoids, and androgen signaling, the action of progesterone and its downstream targets are far less understood.
Progesterone has been generally assumed to play an anti-inflammatory role in immune regulation. In fact, the physiological reduction of progesterone prior to menstruation and preceding labor results in a marked influx of inflammatory cells (macrophages, neutrophils, and T cells) into the decidua resembling a local inflammatory response (Hamilton et al., 2012, Hamilton et al., 2013, Jones, 2004 and Shynlova et al., 2008). Moreover, mice with complete deletion of PR (PRKO) were found to have increased immune cell infiltration into the uterus and impaired thymic function (Tibbetts et al., 1999a and Tibbetts et al., 1999b). At the cellular level, PR expression has been demonstrated in a variety of immune cell types indicative of a direct regulation by progesterone (Butts et al., 2008, Gilliver, 2010 and Hughes, 2012). However, these findings do not explain progesterone control of other leukocyte populations that do not express PR in vivo, such as natural killer cells and granulocytes. Therefore, it is likely that paracrine factors such as cytokines and chemokines act as effectors of steroid hormones, thus enabling systemic immune modulation in the absence of leukocyte steroid receptors. In fact, there is ample evidence in the literature for regulation of immune function by progesterone through its effect on smooth muscle, stromal, and perivascular cells (Gotkin et al., 2006, Hardy et al., 2006, Luk et al., 2010, Shields et al., 2005 and Shynlova et al., 2008). Due to its multiple cellular targets, a comprehensive dissection of cell specific signaling, as well as direct downstream targets of PR, is necessary to understand the multiple immune-modulatory functions of progesterone.
The endothelium is an active participant in immune cell trafficking and is an important barrier in the regulation of leukocyte extravasation into tissues (Ley et al., 2007 and Pober and Sessa, 2007). Upon activation by an inflammatory stimulus, endothelial cells acquire new capabilities including cytokines/chemokine secretion and the expression of endothelial–leukocyte adhesion molecules (Pober and Sessa, 2007). Several reports have demonstrated expression of PR within different human vascular beds (Ingegno et al., 1988, Iruela-Arispe et al., 1999, Krikun et al., 2005, Maybin and Duncan, 2004, Perrot-Applanat et al., 1995 and Rodríguez-Manzaneque et al., 2000), including endothelial cells of human atherosclerotic vessels (Vázquez et al., 1999). Functionally, progesterone has been found to mediate endothelial cell proliferation, transcriptional repression of endothelial–leukocyte adhesion molecules, as well as MMP secretion (Otsuki et al., 2001, Rodríguez-Manzaneque et al., 2000 and Vázquez et al., 1999) implicating a direct function of progesterone in the endothelium. Therefore, we hypothesized that progesterone signaling may modulate the immune system by transcriptionally altering endothelial cell activation and expression of immunomodulatory factors.
Here we provide evidence that PR signaling in the endothelium directly regulates cytokine expression both under physiological conditions, as well as following an acute inflammatory stimulus. PR is able to selectively and directly target a cohort of endothelial cytokines resulting in transcriptional repression and reduction in protein levels by the endothelium. These findings expand our understanding of the cell specific function of progesterone in the endothelium and its potential role in immune regulation through direct mediation of cytokine production.
2. Materials and methods
2.1. Virus production and transduction
Human PR cDNA was PCR amplified and cloned into a lentiviral vector using the following primers with attached restriction site sequences: 5′-PR-Xbal (GCTATCTAGAATGACTGAGCTGAAGGCA) and 3′-PR-STOP-EcoRI (GCTAGAATTCCTACTTTTTATGAAAGAGAAG). Lentivirus-based vectors encoding PR cDNA were generated by transient cotransfection of 293 T cells with a three-plasmid combination, as described previously, with slight modifications (Naldini et al., 1996). The construct pMD.G was used for the production of the VSV-G viral envelope in combination with the packaging constructs pMDLg/pRRE and pRSV-REV, whereas the pRRL constructions correspond to the different transfer vectors. Briefly, 100 mm dishes of nonconfluent 293 T cells were co-transfected with 6.5 µg of pMDLg/pRRE, 3.5 µg of pMDG (encoding the VSV-G envelope), 2.5 µg of pRSV-REV and 10 µg of pRRL-hPR, by the CaPi-DNA coprecipitation method (Chen and Okayama, 1987 and Sakoda et al., 1992). The plasmid vectors were provided by Dr Luigi Naldini (University of Torino, Italy). Next day, the medium was adjusted to make a final concentration of 10 mM sodium butyrate and the cells were incubated for 8 h to obtain high-titer virus production as previously described (Sakoda et al., 1999). Conditioned medium was harvested 16 h later and passed through 0.45 mm filters. Viral titer was determined by assessing viral p24 antigen concentration by ELISA (the Alliance® HIV-I p24 ELISA Kit, Perkin Elmer) and hereafter expressed as µg of p24 equivalent units per milliliter.
2.2. Cell culture
Human umbilical vein endothelial cells were cultured in MCDB-131 media (VEC Technologies, Rensselaer, NY) supplemented with charcoal stripped fetal bovine serum (Omega Scientific, Tarzana, CA). For bead-based multiplex cytokine arrays, HUVECs were grown to confluence in 48 well plates and treated with LPS (1 µM; 0111:B4; Sigma, St. Louis, MO) and/or progesterone (100 nM; Sigma, St. Louis, MO) for 4, 8, and 24 h. Media without serum were collected, and run in triplicate on a 42-plex array analyzed by Eve Technologies. For immunocytochemistry, HUVECs were seeded onto Lab-Tek II 8-well chamber slides (Thermo Scientific, Rochester, NY) and fixed with 4% paraformaldehyde. Cells were probed with an antibody against PR (1:400; clone SP2, Lab Vision, Kalamazoo, MI) followed by an Alexa Fluor secondary (1:300, Invitrogen, Grand Island, NY). Nuclei were stained using 4′,6-diamidino-2-phenylindole (DAPI, 1:1000; Invitrogen, Grand Island, NY). Images were acquired using a Zeiss LSM 520 multiphoton microscope (Zeiss, Germany).
2.3. Immunoblotting
Total HUVEC lysates were resolved by SDS-PAGE, and nitrocellulose membranes (Optitran BA-S 83; Dassel, Germany) were incubated overnight with an anti-PR antibody (1:2000; clone SP2, Lab Vision, Kalamazoo, MI) and anti-GAPDH antibody (1:1000, Millipore, Billerica, MA). Blots were incubated with HRP-conjugated secondary (1:5000; Bio-Rad Laboratories, Hercules, CA) and developed using Supersignal West Pico Chemiluminescent Substrate (Thermo Scientific, Kalamazoo, MI). A Bio-Rad ChemiDoc XRS + and accompanying Image Lab software was used for detection (Bio-Rad Laboratories, Hercules, CA).
2.4. RNA isolation, qPCR, and library preparation
Total RNA was extracted using RNeasy Kit (Qiagen, Valencia, CA). RNA was reverse transcribed using SuperScript First-strand Synthesis System (Invitrogen, Grand Island, NY). qPCR was performed using SYBR Green reagent (Qiagen, Valencia, CA) and PCR products were run on an Opticon2 PCR machine (MJ Research; BioRad, Hercules, CA). Libraries for RNA-sequencing were generated using an Illumina Multiplex System (Illumina, San Diego, CA) and sequenced using HIseq-2000 (Illumina, San Diego, CA). RNA-seq datasets have been deposited in the NCBI Gene Expression Omnibus with the accession number GSE46502.
2.5. RNA-seq analysis
Multiplex runs were debarcoded by in house Unix shell script. Reads were aligned to the human genome (hg19) using TopHat v2.0.4 (Trapnell et al., 2009) and processed with Cufflinks v2.0.1 (Trapnell et al., 2010). Assemblies for all samples were merged using CuffMerge and pairwise differential expression was assessed using Cuffdiff. Genes with a p-value smaller than 0.01 where considered significant. Heatmaps with relative expression were generated by visualizing the log2 values of each gene rpkm divided with the average rpkm of all samples using Java treeview (de Hoon et al., 2004).
2.6. ChIP-sequencing and analysis
HUVECs were infected with hPR lentivirus, grown to confluence, and treated with progesterone for 1 h. For each condition (non-infected-negative control, PR + P, PR only, and IgG control) 10 × 106 cultured HUVECs were used per IP. Cells were crosslinked with 1% formaldehyde, resuspended in 400 µL of lysis buffer (1% SDS, 20 mM EDTA and 50 mM Tris–HCl (pH 8.0)) containing protease inhibitors (Roche, Indianapolis, IN), and sonicated to achieve 200 bp fragments. Samples were immunoprecipitated with 3 µg of anti-PR or IgG antibody. Protein A Dynabeads (Invitrogen, Grand Island, NY) were used to isolate antibody–PR complexes and eluted using 50 mM Tris–HCl, pH 8.0. Crosslinks were reversed by incubation at 65 °C and DNA was purified using Qiagen MinElute Columns. Libraries were generated using Ovation Ultralow IL Multiplex System 1–8 (Nugen, San Carlos, CA) and sequenced using HIseq-2000 (Illumina, San Diego, CA). ChIP-seq data sets have been deposited in the NCBI Gene Expression Omnibus with the accession number GSE43786.
Multiplex runs were debarcoded using Fastx toolkit (http://hannonlab.cshl.edu/fastx_toolkit) and reads were mapped to the human genome (hg19) using bowtie v0.12.7 (Langmead et al., 2009). 12–22 million uniquely mapped reads were obtained for each sample. Peak identification was performed with MACS v1.3.7.1 (Zhang et al., 2008). Peaks were called by comparing peaks in PR and PR + P conditions to that of the input, negative control (non-infected cells) or IgG control. Only peaks that appeared in all three comparisons were determined to be noteworthy. Genes potentially regulated by PR were determined by mapping peaks to nearby genes within 200 kb of the transcriptional start site using the Genomic Regions Enrichment of Annotations Tool (McLean et al., 2010). Intersection of PR binding associated genes with differentially expressed genes was performed using Unix shell scripts.
2.7. Statistical analysis
All data were analyzed using a Student unpaired two-tailed t-test. p-Values less than 0.05 were considered to be statistically significant.
3. Results
3.1. Generation and validation of a lentivirus for expression of human PR
A detailed, comprehensive histological examination of PR expression in the mouse confirmed the presence of PR in the endothelium (Fig. 1A). Interestingly, PR positive endothelial cells were restricted to veins, but absent from arteries. Although expressed by different vascular beds in humans, endothelial PR expression in the mouse was restricted to the vasculature of the uterus, suggestive of its unique importance in this organ.
Fig. 1.
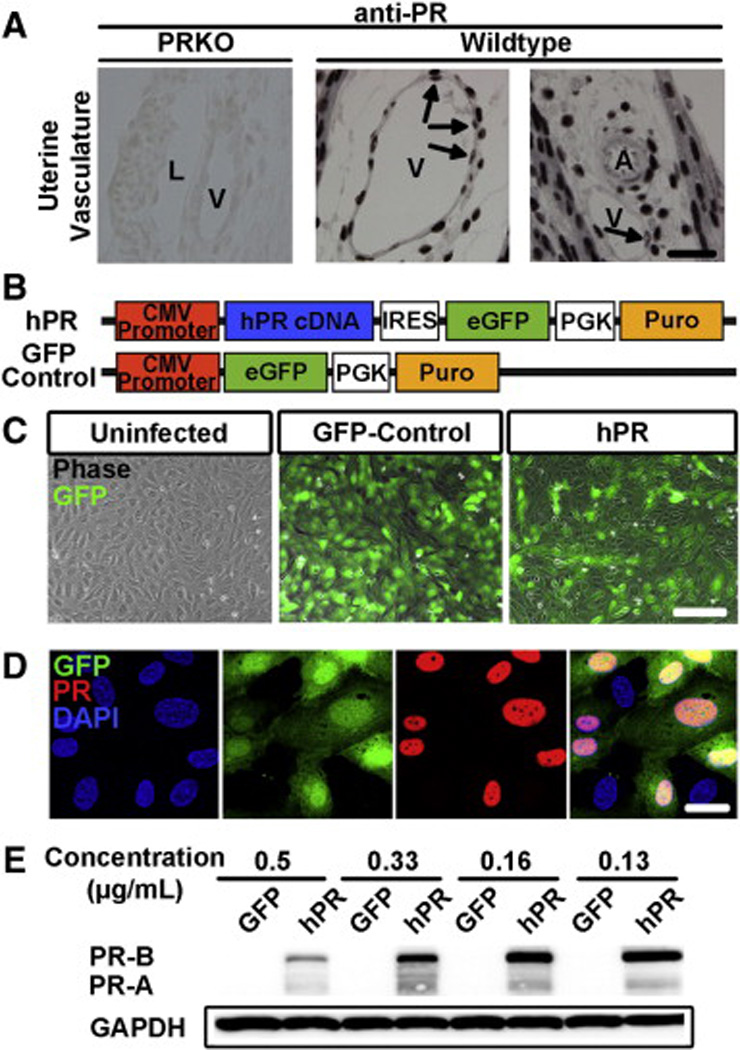
Generation of a lentivirus for human PR expression.(A) Histological sections demonstrate PR expression in the endothelium of veins (V) but not arteries (A) in murine uterine vasculature. PRKO tissue was used as a negative control. Arrows indicate PR positive endothelial cells. L = lymphatics Scale = 25 µm. (B) Scheme depicting human PR (hPR) and GFP control lentiviral constructs. PR cDNA was cloned directly following a CMV promoter. eGFP and puromycin resistance were used to determine infection efficiency and confer selection, respectively. (C) Human umbilical vein endothelial cells infected with a GFP-control or hPR lentivirus. GFP (green) and phase images were superimposed to determine infection efficiency. Scale bar = 50 µm. (D) Confirmation that GFP expression (green) correlates with PR expression (red). DAPI (blue) marks cell nuclei. Scale bar = 20 µm. (E) Western blot analysis of total protein levels from GFP control or hPR infected HUVECs at various viral concentrations. GAPDH was used as a loading control. In all panels, results are representative of 3–5 independent experiments.
In order to gain a better molecular understanding of progesterone function in the endothelium, we overexpressed human PR in human umbilical vein endothelial cells (HUVECs) using lentiviral infection. Full-length human PR cDNA (hPR) was cloned downstream of a CMV promoter and preceding sequences for eGFP and puromycin resistance (Fig. 1B). A lentivirus expressing eGFP under the control of a CMV promoter was used as a control. HUVECs overexpressing the GFP or hPR construct looked morphologically normal when compared to uninfected HUVECs (Fig. 1C). PR protein colocalized with GFP positive cells, confirming GFP as an indicator of PR expressing cells (Fig. 1D). Western blot analysis demonstrated the expression of both PR isoforms, PR-A and PR-B, in HUVECs expressing hPR, but not in cells expressing GFP alone (Fig. 1E). PR protein levels were most optimally expressed at viral concentrations between 0.16 and 0.13 µg/mL, while higher concentrations led to cell death and reduction in PR protein expression.
HUVECs expressing hPR were treated with progesterone to evaluate the ability of the transduced receptor to respond to progesterone (Fig. 2A). Consistent with previous reports, PR was mainly localized to the nucleus in both the presence and absence of progesterone, but was almost exclusively localized to the nucleus following progesterone treatment (Fig. 2A). To determine the optimal infection efficiency of the hPR lentivirus, cells were infected with progressively lower viral concentrations. Titration of virus demonstrated that concentrations less than 0.16 µg/mL led to a reduction of PR expressing cells upon quantification (Fig. 2B). Therefore, all subsequent experiments were performed at an hPR lentiviral concentration of 0.16 µg/mL.
Fig. 2.
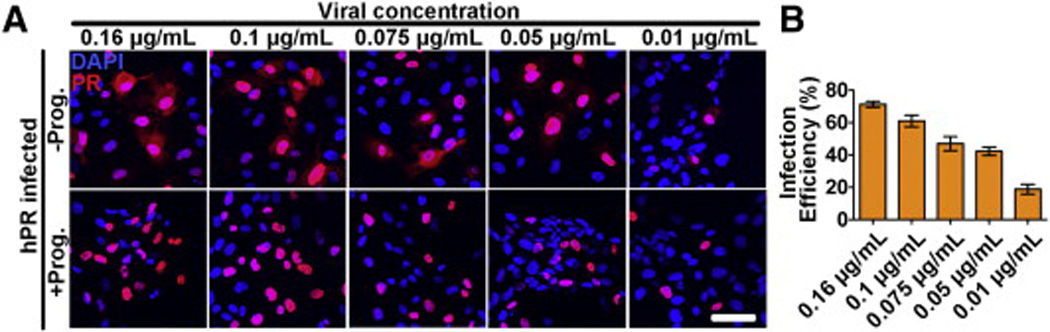
Validation of hPR responsiveness to progesterone.(A) HUVECs overexpressing hPR were treated with or without progesterone (100 nM) for 1 h. PR (red) localization was exclusively confined to the nucleus in the presence of progesterone. DAPI (blue) marks cell nuclei. Scale bar = 50 µm. (B) Infection efficiency of the hPR lentivirus at several different viral concentrations. Efficiency was determined by dividing PR positive cells (red), by total number of nuclei (DAPI, blue) in 10 independent fields from three biological replicates.
3.2. PR negatively regulates endothelial cytokine production
Using next generation RNA sequencing, we explored whether PR signaling may transcriptionally alter the expression of cytokines by the endothelium. First, we assessed which cytokines might be specifically altered when lipopolysaccharide (LPS) was applied to endothelial cultures for 4 and 8 h. A total of 70 cytokines were included in the initial analysis. Of these 70, only 27 showed transcript expression by the endothelium (Fig. 3A), and only 15 of these were significantly (p < 0.01) altered in the presence of LPS at both 4 and 8 h of treatment (Fig. 3B). To determine if progesterone altered the expression of these 15 genes, we examined fold change expression between HUVECs in the presence of LPS alone and those treated concurrently with LPS and progesterone (Fig. 3B). Although majority of these cytokines were downregulated by progesterone, only five: including CCL2, IL-6, IL-8, CXCL1 and CXCL2, were considered to be statistically significant (p < 0.01). To assess whether progesterone alone, in the absence of LPS, was able to reduce the expression of these same five genes, we compared fold change expression between hPR infected cells in the presence or absence of progesterone for 4 h (Fig. 3C). Even in the absence of LPS, progesterone still negatively regulated the expression of IL-8, IL-6, CXCL1, and CXCL1/2, suggesting that progesterone may modulate cytokine production in the absence of an acute inflammatory stimulus.
Fig. 3.
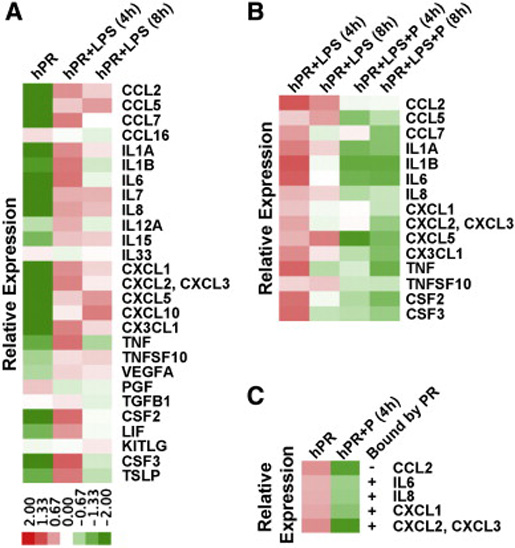
PR regulation of cytokine and chemokine expression.(A) Heat map depicting differential expression of cytokine and chemokines strongly regulated by LPS stimulation of HUVECs for 4 and 8 h compared to nontreated cells. (B) Heat map comparing differential gene expression between LPS treatment in the presence or absence of progesterone. Genes included those found to be significantly (p < 0.01) upregulated by LPS treatment as determined from the heat map in panel A. (C) Heat map comparing differential gene expression between HUVECs treated with or without progesterone. Genes analyzed were those that were significantly (p < 0.01) downregulated by progesterone in the heat map from panel B. + symbolizes genes that were found to be directly bound by PR by ChIP-seq analysis
3.3. PR binding peaks reveal direct transcriptional regulation of cytokine production
To further explore whether PR could directly regulate cytokine expression, we obtained a global read-out of PR binding sites in the HUVEC genome using ChIP-sequencing. Activation of the receptor by progesterone resulted in 9906 PR binding sites. To identify whether the 5 cytokines significantly regulated by progesterone might be direct targets of PR, it was necessary to combine the ChIP-seq and RNA-seq datasets. RNA-seq analysis of HUVECs yielded 431 downregulated genes with a p-value less than 0.01. These genes were then intersected with the list of 3886 genes predicted as regulated by the PR binding sites obtained from ChIP-seq evaluation. The analysis revealed 214 genes that were likely directly repressed by PR. Of this list, 4 out of the 5 cytokines found to be downregulated by progesterone were also predicted to be direct targets of PR (Fig. 3C).
3.4. PR negatively regulates cytokine production by the endothelium
To confirm whether treatment of the endothelium with progesterone leads to a reduction in cytokine secretion, we performed a 42-multiplex bead-based cytokine array. This approach was set up to determine whether reduced RNA transcript levels correlated with cytokine protein production. Of the 42 cytokines analyzed, only 24 were at sufficiently high levels to be detected by the array (Table 1). Analysis of these 24 cytokines uncovered selective regulation of 8 by progesterone, including fractalkine, GRO, IL-6, IL-8, IP-10, MCP-1, PDGF-AA, and PDGFAB/BB. Cytokine regulation was PR-dependent as per evaluation of HUVECs transduced with a GFP control construct. These findings were very similar to the RNA- and ChIP-seq analyses, as IL-6, IL-8, CCL2/MCP-1, and CXCL1/GRO were all found to be targets of PR (Fig. 3C).
Table 1.
Effect of progesterone on cytokine/chemokine secretion by endothelial cells.a
| Cytokine | + LPS/P | Cytokine | + LPS/P |
|---|---|---|---|
| EGF | – | IL-12(p70) | – |
| Eotaxin | – | IL-13 | – |
| FGF-2 | – | IL-15 | – |
| Flt-3 | ND | IL-17 | ND |
| Fractalkine | ↓ | IP-10 | ↓ |
| G-CSF | – | MCP-1 | ↓ |
| GM-CSF | – | MCP-3 | – |
| GRO(Pan) | ↓ | MDC | – |
| INF-alpha2 | – | MIP-1alpha | ND |
| IL-1beta | ND | MIP-1beta | ND |
| IL-1ra | – | PDGF-AA | ↓ |
| IL-2 | ND | PDGFAB/BB | ↓ |
| IL-3 | ND | RANTES | – |
| IL-4 | ND | CD40L | ND |
| IL-5 | ND | siL-2Ralpha | ND |
| IL-6 | ↓ | TGFalpha | ND |
| IL-7 | ND | TNFbeta | ND |
| IL-8 | ↓ | VEGFA | – |
| IL-9 | ND | IFN-gamma | ND |
| IL-10 | ND | IL-1alpha | – |
| IL-12(p40) | – | TNFalpha(78) | ND |
| TNFalpha(80) | ND |
Samples were run using Multiplexing LASER Bead Technology based on uniquely colored bead sets able to recognize up to 100 analytes per well. Human 42-plex 96 well plates were used to simultaneously detect 42 different cytokine/chemokines per sample. Cell culture supernatant from LPS and LPS/P treated HUVECs were run in duplicate and average concentrations were calculated by comparing the fluorescent intensity of each analyte to a cytokine/chemokine specific standard curve (0.64 pg/mL–10,000 pg/mL). Analyte sensitivities were 0.1 pg/mL–30 pg/mL with most being in the 0.1 pg/mL–1 pg/mL range. ND = non-detectable; – = no change; ↓ = decreased analyte concentration.
3.5. Confirmation of cytokine transcript levels following progesterone treatment
In order to confirm the findings from the global transcriptome analysis and the multiplex cytokine array, we more closely examined protein and transcript levels following concurrent exposure of HUVECs to progesterone and LPS (Fig. 4, Fig. 5 and Fig. 6). As predicted, the protein levels for IL-6 were significantly reduced in the presence of progesterone (Fig. 4A). This correlated with a significant decrease in transcript levels as early as 1 h after progesterone treatment (Fig. 4B).
Fig. 4.
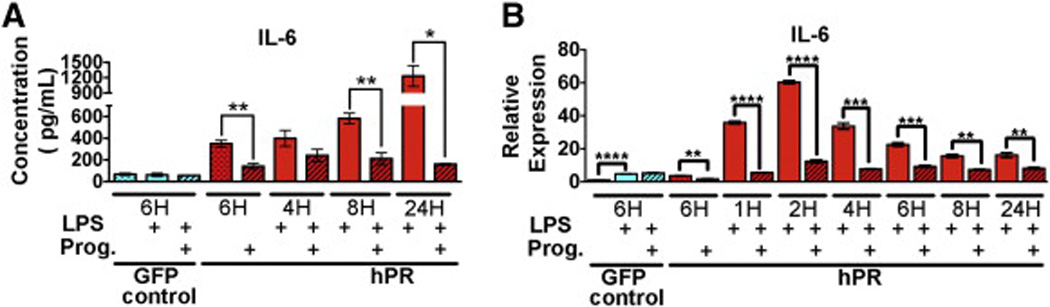
IL-6 repression by progesterone stimulation.(A) Protein expression (pg/µL) of IL-6 determined by cytokine array. HUVECs were treated with LPS (solid bars) or in combination with progesterone (hatched bars) for the indicated times. Graphs depict an average of three biological replicates. *p < 0.01, **p < 0.001. (B) qPCR confirmation of IL-6 expression. HUVECs were treated with LPS (solid bars) or in combination with progesterone (hatched bars) for the indicated times. Ct values were normalized to GAPDH and made relative to PR infected HUVECs in the absence of both LPS and progesterone (red hatched bar). GFP infected cells (first three bars) were used as a control and did not respond to progesterone. Graphs depict an average of three biological replicates run in triplicate. *p < 0.01, **p < 0.001, ***p < 0.0001, and ****p < 0.00001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
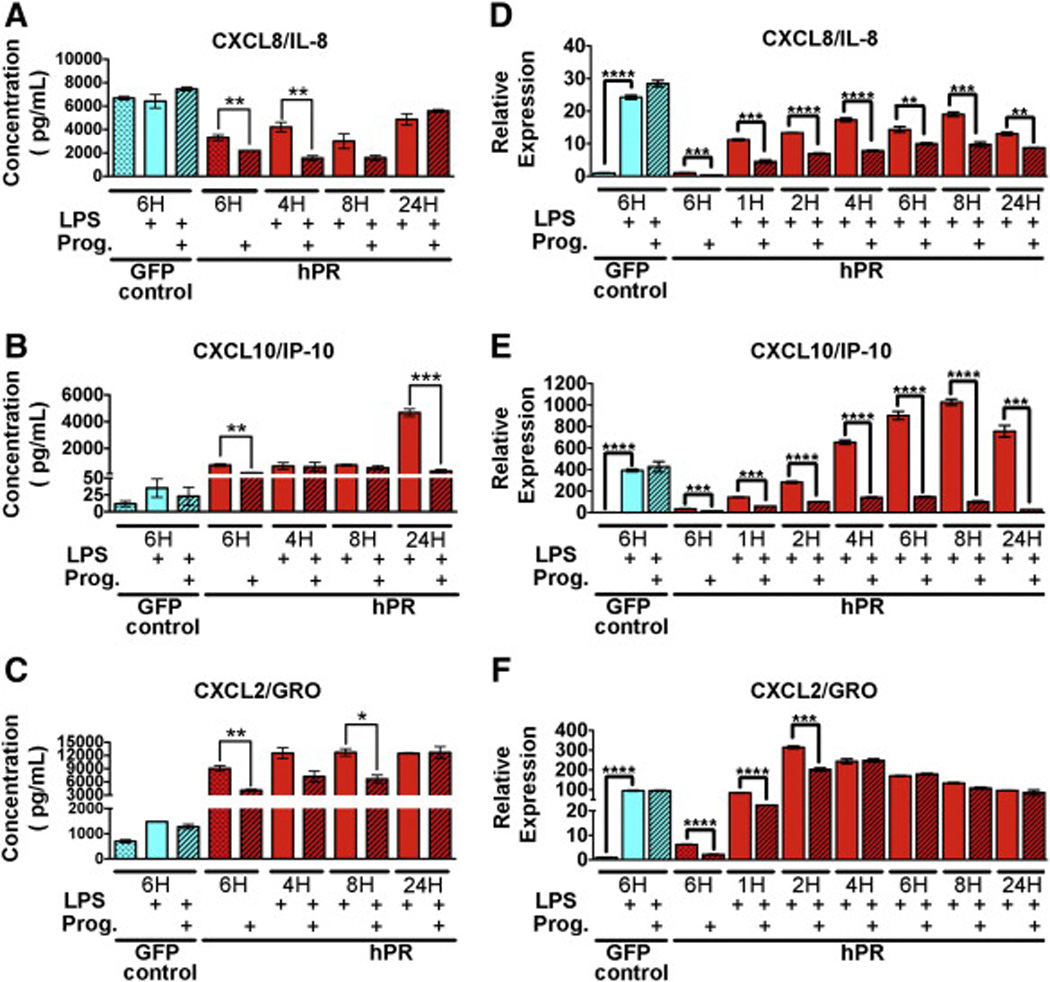
Progesterone regulation of CXC chemokine family members.(A-C) Protein expression (pg/µL) of CXCL8/IL-8 (A) CXCL10/IP-10 (B) and CXCL2/GRO (C) as determined by cytokine array. HUVECs were treated with LPS (solid bars) or in combination with progesterone (hatched bars) for the indicated times. Graphs depict an average of three biological replicates. *p < 0.01, **p < 0.001, ***p < 0.0001. (D-F) qPCR confirmation of CXCL8/IL-8 (D) CXCL10/IP-10 (E) and CXCL2/GRO (F). HUVECs were treated with LPS (solid bars) or in combination with progesterone (hatched bars) for the indicated times. Ct values were normalized to GAPDH and made relative to PR infected HUVECs in the absence of both LPS and progesterone (red dotted bar). GFP infected cells (first three bars) were used as a control and did not respond to progesterone. Graphs depict an average of three biological replicates run in triplicate. ***p < 0.0001, ****p < 0.00001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
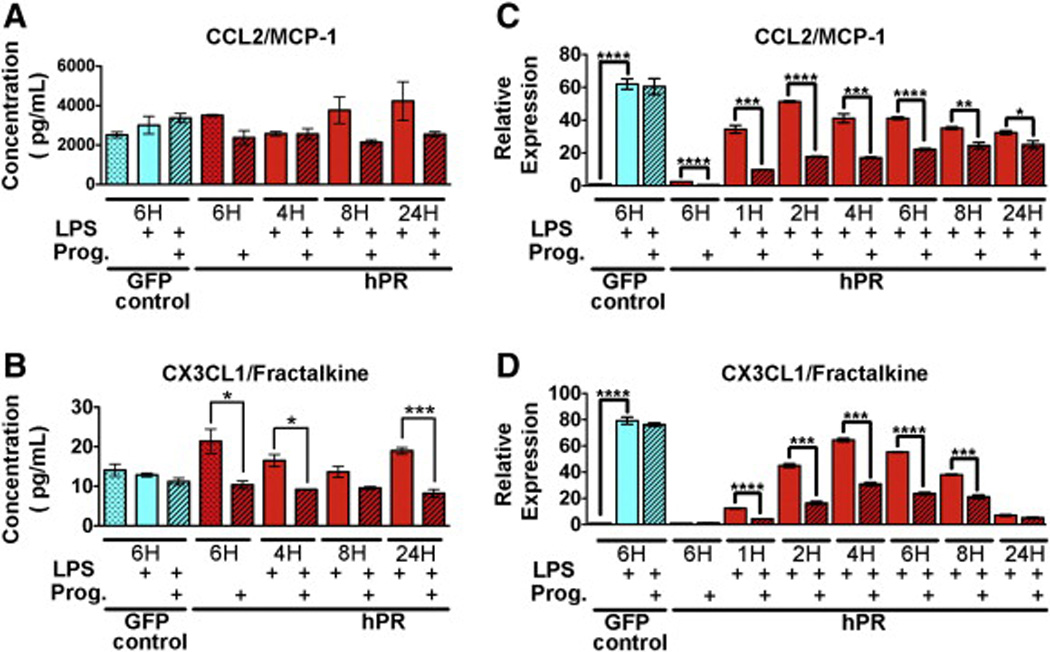
Progesterone regulation of the CC and CX3C family members.(A,B) Protein expression (pg/µL) of CCL2 (A) and CX3CL1/fractalkine (B) as determined by cytokine array. HUVECs were treated with LPS (solid bars) or in combination with progesterone (hatched bars) for the indicated times. Graphs depict an average of three biological replicates. (C,D) qPCR confirmation of CCL2 (C) and CX3CL1 (D). HUVECs were treated with LPS (solid bars) or in combination with progesterone (hatched bars) for the indicated times. Ct values were normalized to GAPDH and made relative to PR infected HUVECs in the absence of both LPS and progesterone (red dotted bar). GFP infected cells (first three bars)were used as a control and did not respond to progesterone. Graphs depict an average of three biological replicates run in triplicate. *p < 0.01, **p < 0.001, ***p < 0.0001, ****p < 0.00001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
IL-8/CXCL8 showed a similar expression pattern to that of IL-6, but regulation by progesterone was not as pronounced (Fig. 5A, D). CXCL10/IP-10 RNA levels were negatively regulated by progesterone at early time points, yet protein levels were not significantly reduced until 24 h after treatment (Fig. 5B, E). Interestingly, transcript levels of CXCL1/GRO were significantly downregulated by progesterone at very early time points of 1 and 2 h (Fig. 5F). This correlated with a reduction in protein at 4 and 8 h, yet this phenomenon did not extend to 24 h (Fig. 5C).
Progesterone significantly reduced RNA levels of the CCL family member, MCP-1/CCL2, yet decreased protein levels did not reach significance (Fig. 6A, C). Alternatively, the CX3C family member, CX3CL1/fractalkine, although not found to be significantly reduced by RNA-seq analysis, did show significant reduction in both RNA and protein expression at all times of progesterone treatment (Fig. 6B, D).
4. Discussion
Hormones are believed to play an important role in the sexual dimorphism underlying diseases with immune etiologies (Gilliver, 2010, Hughes, 2012 and Martocchia et al., 2011). Clearly interdependence exists between different hormonal signaling pathways in immune regulation, yet this complexity makes it difficult to assess the contributions of individual steroid hormones, particularly in vivo. Although several studies have demonstrated the anti-inflammatory properties of progesterone within the context of its reproductive functions (Challis et al., 2009, Gilliver, 2010, Jones, 2004, King and Critchley, 2010, Red-Horse and Drake, 2004 and van Mourik et al., 2009), little is understood as to its direct cellular and molecular targets with respect to immune regulation. As the vascular endothelium is known to mediate leukocyte homing and selective extravasation, we hypothesized that progesterone signaling might transcriptionally modulate the activation state of the endothelium in response to an acute inflammatory stimulus. Using unbiased global expression analysis we demonstrated that progesterone signaling, via PR, directly suppresses a select group of cytokine and chemokines expressed by the endothelium. This reduction was seen both under physiological and pathological activation by LPS, indicating that endothelial cells are also susceptible to anti-inflammatory regulation by progesterone.
Our results indicate that under homeostatic conditions, endothelial expression of PR is selective to veins and conspicuously absent from arteries. This exquisite specificity is consistent with the fact that immune trafficking occurs predominantly in venules and lymphatics (Pober and Sessa, 2007). Thus, restricted expression allows spatial regulation of PR function in response to a systemically distributed ligand. Although PR is not constitutively expressed in the endothelium, its focal and sporadic expression may be indicative of a tightly regulated and precisely localized function. Although not much is known of the role of PR in the endothelium, a select group of in vitro studies have shown that PR can inhibit the expression of the endothelial–leukocyte adhesion molecule, VCAM-1, and the cytokines IL-8 and MCP-1 (Okada et al., 1998, Otsuki et al., 2001 and Simoncini, 2004), yet no study has assessed global expression of endothelial genes upon progesterone stimulation.
To examine the contribution of PR in the vascular endothelium at the molecular level, we employed a cell culture based system using HUVECs. Using global transcriptome analysis we examined cytokine production from the endothelium in the presence of LPS. Of 70 cytokines examined, 29 of these displayed altered expression in the presence of LPS, yet only 15 were considered statistically significant. From these, 5 were significantly downregulated by progesterone (IL-8, IL-6, CCL2, CXCL1 and CXCL2/3) while 4 (IL-6, IL-8, CXCL1, and CXCL2) were directly bound by PR. These findings are intriguing as not only does the endothelium itself preferentially produce a unique subset of cytokines in response to LPS, but only a small proportion of these are presumably controlled by PR. Therefore, progesterone may modulate specific leukocyte subsets in response to an acute inflammatory event. Indeed, the majority of the direct cytokine/chemokine targets of progesterone noted in this study were found to be neutrophil/monocyte attractants (Hamilton et al., 2012 and Romano et al., 1997).
Biologically, the local tissue response to withdrawal of progesterone shows many features characteristic of an inflammatory response (Gilliver, 2010, King and Critchley, 2010 and Oertelt-Prigione, 2012). Following progesterone decline in the circulation that precedes menstruation, there is a significant influx of neutrophils, eosinophils, and macrophages into the uterus, which are likely critical for focal inflammatory mediated endometrial repair (Henriet et al., 2012). Analysis of whole decidual tissue has implicated MCP-1, IL-8, IL-6, MDC, fractalkine, eotaxin, and MCP-3 following the decline in progesterone levels that initiate menstruation (Angstwurm et al., 1997, Critchley et al., 1999, Hamilton et al., 2013, Hannan et al., 2004, Jones, 2004, Jones et al., 1997 and Zhang et al., 2000). Moreover, IL-8 and MCP-1 levels as well as monocyte numbers are increased in human decidua from women taking the PR inhibitor, mifepristone (Critchley et al., 1996). Furthermore, the expression of these two cytokines (both in vitro and in vivo) was inhibited by progesterone (Jones et al., 1997, Kelly et al., 1994, Loudon et al., 2003 and Luk et al., 2010).
Similar to menstruation, proinflammatory cytokines also play a central role in the mechanisms of term and inflammation/infection-induced preterm parturition (Hamilton et al., 2013, MacIntyre et al., 2012 and Norman et al., 2007). Cytokines associated with this process also include MCP-1, IL-8 and IL-6, in addition to RANTES, and MIP-B1 (Hamilton et al., 2012, Hamilton et al., 2013 and Robertson et al., 2010). As progesterone is capable of inhibiting the expression of MCP-1, IL-8, and IL-6 in the endothelium, it is possible that the vasculature plays a critical role in maintaining an immunosuppressive environment in the uterus prior to these immune-mediated events. Naturally, other cell types, including stromal and epithelial cells also play key roles in immune regulation (Hamilton et al., 2013).
Interestingly, we determined that GRO/CXCL1/2/3 is a direct target of progesterone in the endothelium, yet a role for GRO has not been revealed with regard to reproductive immune infiltration. Recently, progesterone has been found to inhibit expression of GRO in ovarian and endometrial cancer cells as well as dendritic cells (Kavandi et al., 2012 and Zhao et al., 2013). As GRO is a potent chemoattractant for neutrophils, more so than IL-8, suppression of GRO by progesterone may play an even stronger role in the inhibition of neutrophil trafficking.
To confirm that progesterone mediated changes in RNA expression correlated with differential protein production, we performed an unbiased bead-based multiplex cytokine array. While all of the cytokines determined to be transcriptionally modulated following RNA-seq analysis were regulated at the protein level, two additional cytokines, fractalkine/CX3CL1 and CXCL10/IP-10, were also found to be downregulated following analysis of the multiplex array. Subsequent qPCR analysis confirmed this reduction at the RNA level.
Based on our analysis of the RNA-seq data, CXCL10 did not meet the criteria as being significantly regulated by LPS, and thus was excluded from further evaluation. In addition, although LPS significantly modulated CX3CL1 expression, it was not significantly altered by progesterone. It is likely that these two cytokines are targets of progesterone, but due to the stringent statistical analysis used for our RNA-seq datasets these cytokines were not found to be significant.
5. Conclusion
The results of this study provide detailed insight into the endothelial cell specific role of progesterone signaling in the regulation of cytokine production. PR directly suppresses the expression of a small subset of cytokines both under physiological conditions and following stimulation by LPS. These results confirm PR as an anti-inflammatory agent in the endothelium, with potential for the negative regulation of immune cell trafficking into tissues. Understanding the factors and cell populations that control immune cells will further clarify gender differences in disease as well as dysregulated immune-mediated reproductive processes such as preterm labor.
Acknowledgments
The authors wish to thank the UCLA Vector Core for generation of the hPR lentiviral construct. This study was supported by funds from the National Institutes of Health, NHLBI, RO1HL74455-01 to MLIA, the Ruth L. Kirschstein National Research Service Award (T32HL69766 to LMG), by fellowships from the American Heart Association (AHA-11PRE7300043 to LMG), by the Leukemia & Lymphoma Society Scholar Award (to TO), by the European Union through the European Social Fund (Mobilitas Grant No. MJD284 to TO) and funding from the Iris Cantor-UCLA Women's Health Center Executive Advisory Board. The UCLA Vector Core is supported by JCCC/P30 CA016042 and CURE/P30 DK041301.
Abbreviations
- PR
progesterone receptor
- LPS
lipopolysaccharide
- HUVEC
human umbilical vein endothelial cell
- PRKO
progesterone receptor knockout
- hPR
human progesterone receptor
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
Conflict of interest disclosure
The authors have no conflicting financial interest.
References
- Adams Waldorf KM, Nelson JL. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol. Invest. 2008;37:631–664. doi: 10.1080/08820130802205886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angstwurm MW, Gärtner R, Ziegler-Heitbrock HW. Cyclic plasma IL-6 levels during normal menstrual cycle. Cytokine. 1997;9:370–374. doi: 10.1006/cyto.1996.0178. [DOI] [PubMed] [Google Scholar]
- Butts C, Bowers E, Horn J, Shukair S, Belyavskaya E, Tonelli L, Sternberg E. Inhibitory effects of progesterone differ in dendritic cells from female and male rodents. Gend. Med. 2008;5:434–447. doi: 10.1016/j.genm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and pregnancy. Reprod. Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid. DNA. Mol. Cell. Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HO, Kelly RW, Lea RG, Drudy TA, Jones RL, Baird DT. Sex steroid regulation of leukocyte traffic in human decidua. Hum. Reprod. 1996;11:2257–2262. doi: 10.1093/oxfordjournals.humrep.a019086. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Jones RL, Lea RG, Drudy TA, Kelly RW, Williams AR, Baird DT. Role of inflammatory mediators in human endometrium during progesterone withdrawal and early pregnancy. J. Clin. Endocrinol. Metab. 1999;84:240–248. doi: 10.1210/jcem.84.1.5380. [DOI] [PubMed] [Google Scholar]
- de Hoon MJL, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- Gilliver SC. Sex steroids as inflammatory regulators. J. Steroid Biochem. Mol. Biol. 2010;120:105–115. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Gotkin JL, Celver J, McNutt P, Shields AD, Howard BC, Paonessa DJ, Napolitano PG. Progesterone reduces lipopolysaccharide induced interleukin-6 secretion in fetoplacental chorionic arteries, fractionated cord blood, and maternal mononuclear cells. Am. J. Obstet. Gynecol. 2006;195:1015–1019. doi: 10.1016/j.ajog.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ, Jones RL. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol. Reprod. 2012;86:39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- Hamilton SA, Tower CL, Jones RL. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLoS One. 2013;8:e56946. doi: 10.1371/journal.pone.0056946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan NJ, Jones RL, Critchley HOD, Kovacs GJ, Rogers PAW, Affandi B, Salamonsen LA. Coexpression of fractalkine and its receptor in normal human endometrium and in endometrium from users of progestin-only contraception supports a role for fractalkine in leukocyte recruitment and endometrial remodeling. J. Clin. Endocrinol. Metab. 2004;89:6119–6129. doi: 10.1210/jc.2003-031379. [DOI] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol. Endocrinol. 2006;20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- Henriet P, GaideChevronnay HP, Marbaix E. The endocrine and paracrine control of menstruation. Mol. Cell. Endocrinol. 2012;358:197–207. doi: 10.1016/j.mce.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Hughes GC. Progesterone and autoimmune disease. Autoimmun. Rev. 2012;11:A502–A514. doi: 10.1016/j.autrev.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingegno MD, Money SR, Thelmo W, Greene GL, Davidian M, Jaffe BM, Pertschuk LP. Progesterone receptors in the human heart and great vessels. Lab. Invest. 1988;59:353–356. [PubMed] [Google Scholar]
- Iruela-Arispe ML, Rodriguez-Manzaneque JC, Abu-Jawdeh G. Endometrial endothelial cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. UMIC. 1999;6:127–140. [PubMed] [Google Scholar]
- Jones RL. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J. Clin. Endocrinol. Metab. 2004;89:6155–6167. doi: 10.1210/jc.2004-0507. [DOI] [PubMed] [Google Scholar]
- Jones RL, Kelly RW, Critchley HO. Chemokine and cyclooxygenase-2 expression in human endometrium coincides with leukocyte accumulation. Hum. Reprod. 1997;12:1300–1306. doi: 10.1093/humrep/12.6.1300. [DOI] [PubMed] [Google Scholar]
- Kaushic C, Roth KL, Anipindi V, Xiu F. Increased prevalence of sexually transmitted viral infections in women: the role of female sex hormones in regulating susceptibility and immune responses. J. Reprod. Immunol. 2011;88:204–209. doi: 10.1016/j.jri.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Kavandi L, Collier MA, Nguyen H, Syed V. Progesterone and calcitriol attenuate inflammatory cytokines CXCL1 and CXCL2 in ovarian and endometrial cancer cells. J. Cell. Biochem. 2012;113:3143–3152. doi: 10.1002/jcb.24191. [DOI] [PubMed] [Google Scholar]
- Kelly RW, Illingworth P, Baldie G, Leask R, Brouwer S, Calder AA. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum. Reprod. 1994;9:253–258. doi: 10.1093/oxfordjournals.humrep.a138491. [DOI] [PubMed] [Google Scholar]
- King AE, Critchley HOD. Oestrogen and progesterone regulation of inflammatory processes in the human endometrium. J. Steroid Biochem. Mol. Biol. 2010;120:116–126. doi: 10.1016/j.jsbmb.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Krikun G, Schatz F, Taylor R, Critchley HOD, Rogers PAW, Huang J, Lockwood CJ. Endometrial endothelial cell steroid receptor expression and steroid effects on gene expression. J. Clin. Endocrinol. Metab. 2005;90:1812–1818. doi: 10.1210/jc.2004-1814. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Loudon JAZ, Elliott CL, Hills F, Bennett PR. Progesterone represses interleukin-8 and cyclo-oxygenase-2 in human lower segment fibroblast cells and amnion epithelial cells. Biol. Reprod. 2003;69:331–337. doi: 10.1095/biolreprod.102.013698. [DOI] [PubMed] [Google Scholar]
- Luk J, Seval Y, Ulukus M, Ulukus EC, Arici A, Kayisli UA. Regulation of monocyte chemotactic protein-1 expression in human endometrial endothelial cells by sex steroids: a potential mechanism for leukocyte recruitment in endometriosis. Reprod. Sci. 2010;17:278–287. doi: 10.1177/1933719109352380. [DOI] [PubMed] [Google Scholar]
- MacIntyre DA, Sykes L, Teoh TG, Bennett PR. Prevention of preterm labour via the modulation of inflammatory pathways. J. Matern. Fetal Neonatal Med. 2012;25(Suppl. 1):17–20. doi: 10.3109/14767058.2012.666114. [DOI] [PubMed] [Google Scholar]
- Martocchia A, Stefanelli M, Cola S, Falaschi P. Sex steroids in autoimmune diseases. Curr. Top. Med. Chem. 2011;11:1668–1683. doi: 10.2174/156802611796117595. [DOI] [PubMed] [Google Scholar]
- Maybin JA, Duncan WC. The human corpus luteum: which cells have progesterone receptors? Reproduction. 2004;128:423–431. doi: 10.1530/rep.1.00051. [DOI] [PubMed] [Google Scholar]
- McCombe PA, Greer JM, Mackay IR. Sexual dimorphism in autoimmune disease. Curr. Mol. Med. 2009;9:1058–1079. doi: 10.2174/156652409789839116. [DOI] [PubMed] [Google Scholar]
- McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth. 2007;7(Suppl. 1):S7. doi: 10.1186/1471-2393-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertelt-Prigione S. Immunology and the menstrual cycle. Autoimmun. Rev. 2012;11:A486–A492. doi: 10.1016/j.autrev.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Okada M, Matsumori A, Ono K, Furukawa Y, Shioi T, Iwasaki A, Matsushima K, Sasayama S. Cyclic stretch upregulates production of interleukin-8 and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1998;18:894–901. doi: 10.1161/01.atv.18.6.894. [DOI] [PubMed] [Google Scholar]
- Otsuki M, Saito H, Xu X, Sumitani S, Kouhara H, Kishimoto T, Kasayama S. Progesterone, but not medroxyprogesterone, inhibits vascular cell adhesion molecule-1 expression in human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2001;21:243–248. doi: 10.1161/01.atv.21.2.243. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M, Cohen-Solal K, Milgrom E, Finet M. Progesterone receptor expression in human saphenous veins. Circulation. 1995;92:2975–2983. doi: 10.1161/01.cir.92.10.2975. [DOI] [PubMed] [Google Scholar]
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Drake PM. Human pregnancy: the role of chemokine networks at the fetalmaternal interface. Expert. Rev. Mol. Med. 2004;6:1–14. doi: 10.1017/S1462399404007720. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Christiaens I, Dorian CL, Zaragoza DB, Care AS, Banks AM, Olson DM. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology. 2010;151:3996–4006. doi: 10.1210/en.2010-0063. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Manzaneque JC, Graubert M, Iruela-Arispe ML. Endothelial cell dysfunction following prolonged activation of progesterone receptor. Hum. Reprod. 2000;15(Suppl. 3):39–47. doi: 10.1093/humrep/15.suppl_3.39. [DOI] [PubMed] [Google Scholar]
- Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- Sakoda T, Kaibuchi K, Kishi K, Kishida S, Doi K, Hoshino M, Hattori S, Takai Y. smg/rap1/Krev-1 p21s inhibit the signal pathway to the c-fos promoter/enhancer from c-Ki-ras p21 but not from c-raf-1 kinase in NIH3T3 cells. Oncogene. 1992;7:1705–1711. [PubMed] [Google Scholar]
- Sakoda T, Kasahara N, Hamamori Y, Kedes L. A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J. Mol. Cell. Cardiol. 1999;31:2037–2047. doi: 10.1006/jmcc.1999.1035. [DOI] [PubMed] [Google Scholar]
- Shields AD, Wright J, Paonessa DJ, Gotkin J, Howard BC, Hoeldtke NJ, Napolitano PG. Progesterone modulation of inflammatory cytokine production in a fetoplacental artery explant model. Am. J. Obstet. Gynecol. 2005;193:1144–1148. doi: 10.1016/j.ajog.2005.05.046. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J. Immunol. 2008;181:1470–1479. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- Simoncini T. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology. 2004;145:5745–5756. doi: 10.1210/en.2004-0510. [DOI] [PubMed] [Google Scholar]
- Tibbetts TA, Conneely OM, O'Malley BW. Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol. Reprod. 1999;60:1158–1165. doi: 10.1095/biolreprod60.5.1158. [DOI] [PubMed] [Google Scholar]
- Tibbetts TA, DeMayo F, Rich S, Conneely OM, O'Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12021–12026. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mourik MSM, Macklon NS, Heijnen CJ. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J. Leukoc. Biol. 2009;85:4–19. doi: 10.1189/jlb.0708395. [DOI] [PubMed] [Google Scholar]
- Vázquez F, Rodríguez-Manzaneque JC, Lydon JP, Edwards DP, O'Malley BW, Iruela-Arispe ML. Progesterone regulates proliferation of endothelial cells. J. Biol. Chem. 1999;274:2185–2192. doi: 10.1074/jbc.274.4.2185. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lathbury LJ, Salamonsen LA. Expression of the chemokine eotaxin and its receptor, CCR3, in human endometrium. Biol. Reprod. 2000;62:404–411. doi: 10.1095/biolreprod62.2.404. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Koga K, Osuga Y, Izumi G, Takamura M, Harada M, Hirata T, Hirota Y, Yoshino O, Inoue S, Fujii T, Kozuma S. Cyclic stretch augments production of neutrophil chemokines and matrix metalloproteinases-1 (MMP-1) from human decidual cells, and the production was reduced by progesterone. Am. J. Reprod. Immunol. 2013;69:454–462. doi: 10.1111/aji.12092. [DOI] [PubMed] [Google Scholar]


