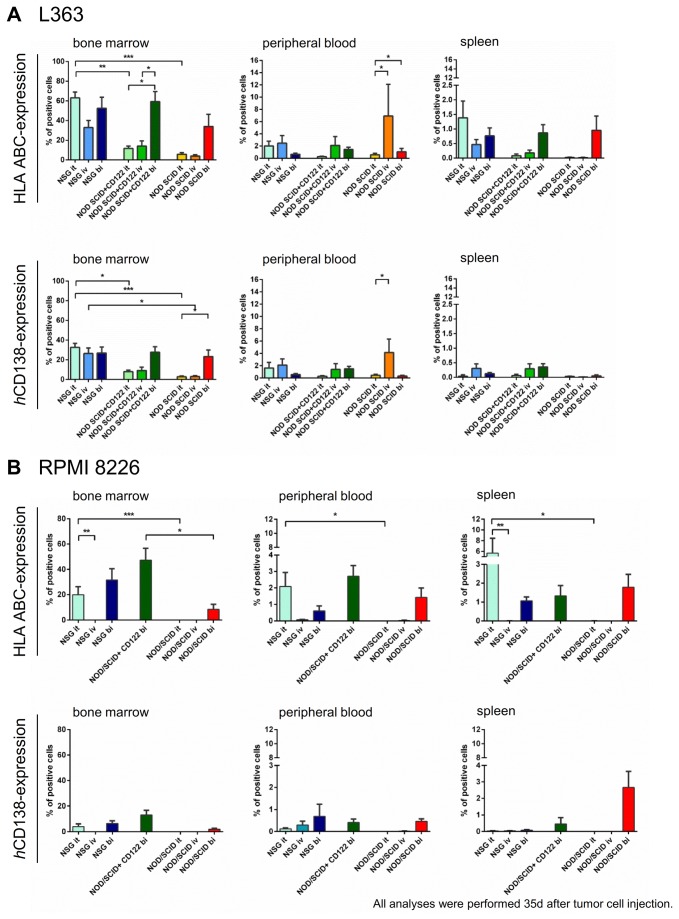
Figure 1. Engraftment of human L363- and human RPMI8226- cells in vivo determined via flow cytometry.
A total of 106 mice received human L363 cells and 49 mice received human RPMI8226 cells via intratibial injection (it, L363: n=36, RPMI8226: n=15), intravenous injection (iv, L363: n=34, RPMI8226: n=10) or subcutaneous long bone implants (bi, L363: n=36, RPMI8226: n=24). Analyses were performed 35 days after tumor cell injection or when mice showed bad overall condition. Lack of NK cell activity, either by use of NSG mice or by use of mCD122-Ab treatment in NOD/SCID mice, and direct contact of implanted tumor cells with the murine bone marrow (BM), after it- or bi-injection, enhanced the engraftment of human MM cells. Human (h)HLA-A,B,C- as compared to hCD138-expression showed higher human tumor cell engraftment. This engraftment was most substantial in the BM, whereas peripheral blood (PB) and spleen engraftment was lower, albeit with similar patterns as in the BM. A. Mean tumor infiltration rates of human L363- cells invivo determined via flow cytometry. In the BM, high human engraftment was obtained in NSG mice, irrespective of the injection modus. This could only be obtained in NOD/SCID mice, if pretreatment with mCD122-Ab and bi-implantation were performed. The PB engraftment after iv-injection of human L363 cells in NOD/SCID mice was most likely induced due to the plasma cell leukemia nature of these cells [28,29]. B. Mean tumor infiltration rates of human RPMI8226- cells invivo determined via flow cytometry. Human RPMI8226 cells displayed similar engraftment patterns compared to L363 cells, albeit to a lower extend and highest BM engraftment potential with bi-injection regardless of the mouse strain. Differences between various conditions did not reach significance for all subgroups due to restricted group sizes.