Table 1.
In vitro Cytotoxicity of (E)-N-Aryl-2-arylethenesulfonamides (6)
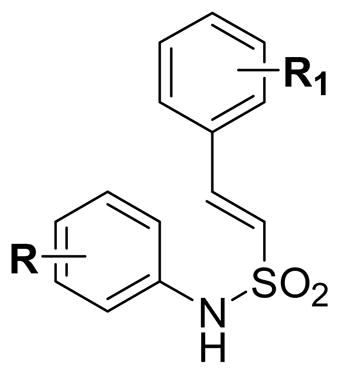
| ||||
|---|---|---|---|---|
| Compd | R | R1 | IC50(μM)a | |
| DU145 | K562 | |||
| 6a | H | H | 10 | 10 |
| 6b | 4-Cl | H | 20 | 20 |
| 6c | 4-F | 4-Br | 10 | 10 |
| 6d | 4-F | 4-OCH3 | 10 | 10 |
| 6e | 4-OCH3 | 2-OCH3 | 5 | 5 |
| 6f | 4-OCH3 | 4-OCH3 | 5 | 5 |
| 6g | 4-OCH3 | 2,4-(OCH3)2 | 15 | 15 |
| 6h | 4-OCH3 | 2,6-(OCH3)2 | 0.375 | 5 |
| 6i | 4-OCH3 | 2,4,6-(OCH3)3 | 0.2 | 0.075 |
| 6j | 4-OCH3 | 3,4,5-(OCH3)3 | 35 | 35 |
| 6k | 2,4,6-(OCH3)3 | 4-OCH3 | 7.5 | 2.5 |
| 6l | 4-OCH3 | 2,6-(OCH3)2,4-OH | 10 | 10 |
| 6m | 4-OCH3 | 2,4,6-F3 | 75 | 15 |
| 6n | 4-OCH3 | 2,3,4,5,6-F5 | 10 | 5.0 |
| 6o | 3-F,4-OCH3 | 2,4,6-(OCH3)3 | 0.2 | 0.075 |
| 6p | 3-OH,4-OCH3 | 2,4,6-(OCH3)3 | 0.03 | 0.015 |
| 6q | 3-OCOCH2C(CH3)2-C6H(CH3)2O2, 4-OCH3 | 2,4,6-(OCH3)3 | 0.009 | 0.008 |
| 6r | 3-OPO(ONa)2,4-OCH3 | 2,4,6-(OCH3)3 | 0.04 | 0.0075 |
| 6s | 3-NO2,4-OCH3 | 2,4,6-(OCH3)3 | 2.5 | 1.0 |
| 6t | 3-NH2,4-OCH3 | 2,4,6-(OCH3)3 | 0.005 | 0.003 |
| 6u | 3-NO2,4-OCH3 | 3,4,5-(OCH3)3 | 75 | 75 |
| 6v | 3-NH2,4-OCH3 | 3,4,5-(OCH3)3 | 35 | 15 |
| 6w | 3-NO2,4-OCH3 | 2,6-(OCH3)2,4-O(CH2)3COOH | 100 | 100 |
| 6x | 3-NH2,4-OCH3 | 2,6-(OCH3)2,4-O(CH2)3COOH | 10 | 10 |
| 6y | 3-OH,4-OCH3 | 2,6-(OCH3)2,4-O(CH2)3COOH | 10 | 10 |
| 6z | 3-NO2,4-F | 2,4,6-(OCH3)3 | 100 | 75 |
| 6aa | 3-NH2,4-F | 2,4,6-(OCH3)3 | 10 | 5 |
| 6ab | 3,5-(NO2)2,4-OCH3 | 2,4,6-(OCH3)3 | 10 | 10 |
| 6ac | 3,5-(NH2)2,4-OCH3 | 2,4,6-(OCH3)3 | 2.5 | 7.5 |
| 6ad | 3-F,4-OCH3 | 4-OCH3 | 5 | 1 |
| 6ae | 3-F,4-OCH3 | 2,3,4,5,6-F5 | 10 | 10 |
| 6af | 3-NO2,4-OCH3 | 2,3,4,5,6-F5 | 100 | 100 |
| 6ag | 3-NH2,4-OCH3 | 2,3,4,5,6-F5 | 75 | 75 |
| 6ah | 2,3,4,5,6-F5 | 3-NO2,4-OCH3 | 100 | 75 |
| 6ai | 2,3,4,5,6-F5 | 3-NH2,4-OCH3 | 35 | 35 |
| 6aj | 2,3,4,5,6-F5 | 2,3,4,5,6-F5 | 35 | 35 |
IC50 values are the compound concentrations (μM) required to inhibit cell proliferation by 50% of tumor cells following 96 h treatment with the tested compound; values represent the mean SD from the dose response curves of two independent experiments and are within 5–10%.
