Abstract
Objective
To investigate the phytochemical screening (group determination) and selected pharmacological activities (antioxidant, antimicrobial and analgesic activity) of the plant Sida cordifolia Linn (S. cordifolia).
Methods
Eighty percent concentrated ethanol extract of the roots was used. To identify the chemical constituents of plant extract standard procedures were followed. In phytochemical screening the crude extract was tested for the presence of different chemical groups like reducing sugar, tannins, saponins, steroids, flavonoids, gums, alkaloids and glycosides. The antioxidant property of ethanolic extract of S. cordifolia was assessed by DPPH free radical scavenging activity. Analgesic activity of the extract was tested using the model of acetic acid induced writhing in mice. Diclofenac sodium is used as reference standard drug for the analgesic activity test. Antibacterial activity of plant extract was carried out using disc diffusion method with five pathogenic bacteria comparison with kanamycin as a standard.
Results
Phytochemical analysis of the ethanolic extract of the roots of S. cordifolia indicated the presence of reducing sugar, alkaloids, steroids and saponins. In DPPH scavenging assay the IC50 value was found to be 50 µg/mL which was not comparable to the standard ascorbic acid. The crude extract produced 44.30% inhibition of writhing at the dose of 500 mg/kg body weight which is statistically significant (P>0.001). The in vitro antimicrobial activity of the ethanol extract of the roots of S. cordifolia showed no antimicrobial activity against five types of microorganisms. The experiment was conducted only with five species of bacteria as test species, which do not at all indicate the total inactivity against micro-organisms.
Conclusions
The obtained results provide a support for the use of this plant in traditional medicine but further pharmacological studies are required.
Keywords: Antioxidant, Antimicrobial, Analgesic, DPPH, Phytochemical screening
1. Introduction
About 25% of prescribed drugs in the world are of plant origin[1]. Approximately 80% people rely on traditional plant based medicines for their initial health care needs in developing countries[2]. From the ancient period different parts of medicinal plants have been used for ailments caused by microorganisms. There is a wide range of medicinal plant parts possessing a variety of pharmacological activities, such as flowers, leaves, barks, stems, fruits and roots extracts which are used as powerful raw drug. Recently there is a widespread interest of plants derived drugs which reflect its recognition of the validity of many traditional claims regarding the value of natural products in health care[3]. For the quality control of traditional medicine phytochemical screening is mainly applied. Nowadays, secondary plant metabolites previously with unknown pharmacological activities have been extensively investigated as a source of medicinal agents[4]. Thus it is anticipated that phytochemicals with enough antibacterial efficacy will be used for the treatment of the bacterial infections[5]. According to WHO, to obtain a variety of new herbal drugs, medicinal plants are the best sources. Therefore, in order to determine the potential use of herbal medicine, it is important to emphasize the study of medicinal plants that found in folklore[3],[6]. To prevent or deter free radical induced lipid oxidation antioxidants are added to a variety of foods[7]. Continuous exposure to chemicals and contaminants increases the free radicals amount and causes irreversible oxidative damage[8],[9]. Improved antioxidant status plays an important role in minimizing the oxidative damage[10]. Currently, the development of resistance of pathogens against antibiotics has become a difficult issue caused by the uncontrolled use of modern antibiotics[11]–[16]. Furthermore, there are many reports on antibacterial activity of various plants growing in different region[17],[18].
Sida cordifolia Linn. (S. cordifolia), commonly known as berela (Bengali), is an herb under Family: Malvaceae that is extensively used as a common herbal drug in the Indian subcontinent. It is used in ayurvedic medicine[19]; it has anti-inflammatory, anti-cancer, antibacterial activities and has been investigated for encouraging liver re-growth[20]–[24]. It was reported that the water extracts of the leaves possess analgesic and anti-inflammatory activities in animal models[25]. On the central nervous system it has a depressive effect[26]. Moreover, the presence of ephedrine, vasicinol, asicinone and N-methyl tryptophan had been supported by the earlier phytochemical studies on the roots[27]. Recently, studies showed that 50% ethanolic extract of S. cordifolia has got antioxidant and anti-inflammatory potential and the activity was comparable with the standard drug diphenyl[28]. Since, there are insufficient studies on S. cordifolia roots extract and studies must be conducted to determine its activity as medicinal plants, this research work was designed to identify the chemical groups responsible for the traditional use, in addition to studying the analgesic and antibacterial effect of S. cordifolia roots.
2. Materials and methods
2.1. Collection and identification of plant material
For this present investigation the S. cordifolia was collected from Khulna region, Bangladesh in November, 2004. The plant was identified by Bangladesh National Herbarium, Mirpur, Dhaka (Accession nomber is 31116).
2.2. Preparation of the plant material
The collected plant parts (roots) were separated from undesirable materials, plants or plant parts. They were sun-dried for one week. The plant parts were ground into a coarse powder with the help of a suitable grinder. The powder was stored in an airtight container and kept in a cool, dark and dry place until analysis commenced.
2.3. Preparation of plant extract
About 200 g of powdered material was taken in a clean, flat-bottomed glass container and soaked in 800 mL of 95% ethanol. The container with its contents was sealed and kept for a period of 7 d accompanying occasional shaking and stirring. The whole mixture then underwent a coarse filtration by a piece of clean, white cotton material. Then it was filtered through Whatman filter paper (RE200, Bibby Sterilin, UK). The filtrate (ethanol extract) obtained was evaporated under ceiling fan and in a water-bath until dried. It rendered a gummy concentrate of brown color. The gummy concentrate was designated as crude extract or ethanolic extract.
2.4. Experimental animal
Young Swiss albino mice aged 4-5 weeks, average weight 20-28 g were used for the experiment. The mice were purchased from the Animal Research Branch of the International Center for Diarrhoeal Disease and Research, Bangladesh (ICDDRB). They were kept under standard environmental condition for one week for adaptation after the purchase and fed with ICDDRB formulated rodent food and water. The ethical rules published by International Association for the Study of Pain were respected[29].
2.5. Chemicals and reagents
All the chemicals used are of analytical reagent grade. Mercuric iodide, potassium iodide, copper sulphate, sodium potassium tartarate, sodium hydroxide, cupric sulphate, sodium citrate, anhydrous sodium carbonate, naphthol, ferric chloride, lead acetate were obtained from Sigma Chemical Co., USA and concentrated hydrochloric acid, sulfuric acid were obtained from Merck, Germany.
2.6. Phytochemical screening
Testing of different chemical groups present in extract represent the preliminary phytochemical studies. To identify the chemical constituents of plant extract standard procedures are followed. The crude extracts were qualitatively tested for the presence of chemical constituents using the following reagents and chemicals: reducing sugar with Benedict's solution, flavonoids with the use of HCl, tannins with ferric chloride and potassium dichromate solution, saponins with ability to produce stable foam, gums with Molish reagent and sulphuric acid, steroids with sulphuric acid, alkaloids with Mayer's reagent and Dragendroff's reagent and finally color change was observed respectively[30],[31]. In each test 10% (w/v) solution of extract in ethanol was taken unless otherwise mentioned in individual test.
2.6.1. Determination of reducing sugar
Initially 0.5 mL of aqueous extract of the plant material was taken in a test tube. Then 5 mL of Benedict's solution was added to the test tube, boiled for 5 min and allowed to cool spontaneously.
2.6.2. Determination of flavonoids
A few drops of concentrated hydrochloric acid were added to a small amount of an alcoholic extract of the plant material. Immediate development of a red color indicated the presence of flavonoids.
2.6.3. Determination of tannins
At the beginning 5 mL of the extract was taken in a test tube. Then 1 mL of 5% ferric chloride solution was added or 1 mL of 10% potassium dichromate solution was added.
2.6.4. Determination of saponins
At first 1 mL solution of the extract was diluted with distilled water to 20 mL and shaken in a graduated cylinder for 15 min.
2.6.5. Determination of gums
Initially 5 mL solution of the extract was taken and then Molish reagent and sulphuric acid were added.
2.6.6. Determination of steroids
At first 1 mL solution of extract was taken and then added 1 mL sulphuric acid. Red color indicated the presence of steroid.
2.6.7. Determination of alkaloids
At the beginning 2 mL solution of the extract and 0.2 mL of dilute hydrochloric acid were taken in a test tube. Then 1 mL of Mayer's reagent was added. Yellow color precipitate was formed that indicated the presence of alkaloids or 1 mL of Dragendroff's reagent was added. Orange brown precipitate was formed that indicated the presence of alkaloids.
2.7. DPPH radical scavenging activity
The free radical scavenging capacity of the extracts was determined using DPPH[32],[33]. A methanol DPPH solution (0.004% w/v) was mixed with serial dilutions (1 to 500 µg) of extracts and after 30 min, the absorbance was read at 515 nm using a spectrophotometer. The experiment was performed in duplicate and average absorption was noted for each concentrations. Ascorbic acid was used as a standard. The percent inhibitions were plotted against log concentration and from the graph IC50 was calculated.
2.8. Antimicrobial potential
The antimicrobial assay was performed by using the disc diffusion method[34],[35]. Five pathogenic bacteria were used as test organisms for antibacterial activity of sample extract. These organisms were collected from the Microbiology Laboratory of Square Pharmaceutical Limited, Pabna and preserved in Microbiology Lab of Pharmacy Discipline, Khulna University, Khulna. About 500 µg/disc of the sample extract were used to observe the antimicrobial activity and compared with the standard kanamycin (30 µg/disc). The test organisms were inoculated on 10 mL previously sterilized nutrient agar media, mixed thoroughly and transferred immediately to the sterile petri dish in an aseptic condition using a sterile loop. Prepared sample and standard solutions were applied to the corresponding petri dish. The plates were incubated overnight at 37 °C. After proper incubation, clear zone of inhibition around the point of application of sample solution were measured and expressed in mm.
2.9. Analgesic potential
Analgesic activity of the ethanolic extract of S. cordifolia was tested using the model of acetic acid induced writhing in mice[36]. The test consists of injecting 0.7% acetic acid solution and observing the animal for specific contraction of body referred as ‘writhing’. Diclofenac Na was used as reference standard drug. Experimental animals were randomly selected and divided into three groups consisting of 5 mice in each group. Each group received a particular treatment i.e., control, positive control and 1 dose of the extract. Each mouse was weighed properly and the dose of the test samples (500 mg) and control materials (15 mL and 25 mg) were adjusted on the basis of per kg of body weight.
2.9.1. Preparation of test samples and control material
For sample preparation 250 mg of the sample was measured. The extracts were triturated in unidirectional manner with the addition of small amount of Tween-80. After proper mixing of extract and Tween-80, the distilled water was slowly added. The final volume of the suspension was made 5 mL. For positive control 12.5 mg of diclofenac Na was taken and a suspension of 5 mL was made. About 1% Tween-80 solution in distilled water was added to 15 mL to prepare control solution. For preparation of 0.7% acetic acid solution, 0.7 mL glacial acetic acid was mixed with distilled water to 100 mL.
2.9.2. Determination of analgesic activity
Test samples, control and diclofenac Na were given orally by means of a feeding needle. A thirty minutes interval was given to ensure proper absorption of the administered substances. Then the chemical that induces writhing, acetic acid solution (0.7%, 10 mL/kg), was administered orally to each of the animals of a group. After an interval of five minutes, which was given for absorption of acetic acid, number of squirms (writhing) was counted for 15 min. Each mouse of all groups was observed carefully to count the number of writhing that they had made in 15 min.
2.10. Statistical analysis
Statistical analysis for animal experiment was carried out using One-way ANOVA followed by Dunnet's multiple comparisons. The results obtained were compared with the control group. P<0.001 was considered to be statistically significant.
3. Results
3.1. Phytochemical screening
The crude extract was subjected for chemical group tests and identified to have various types of important chemical constituents. Results of different chemical group tests are given in Table 1.
Table 1. Results of phytochemical screening (chemical group tests).
| Secondary metabolite | Name of the test | Observation | Result |
| Reducing sugar | Benedict's test | Red precipitate | ++ |
| Alkaloids | Mayer's test | Yellow color precipitate | ++ |
| Hager's test | Orange brown precipitate | ++ | |
| Flavonoids | General test | No red coloration | - - |
| Tannins | FeCl3 test | No brownish green color | - - |
| Saponins | Frothing test | Change was observed | ++ |
| Gums & Carbohydrates | Molisch Test | No red-violet layer at the interface between the acid (bottom) and aqueous (upper) layers | - - |
| Steroids | Sulphuric acid test | Red color was observed | ++ |
“++” stands for the presence and “--” indicates the absence of secondary metabolites.
From the results it was observed that reducing sugar, saponins, steroids and alkaloids were detected in the ethanolic extract of S. cordifolia roots and other experimental chemicals are absent.
3.2. Antioxidant potential
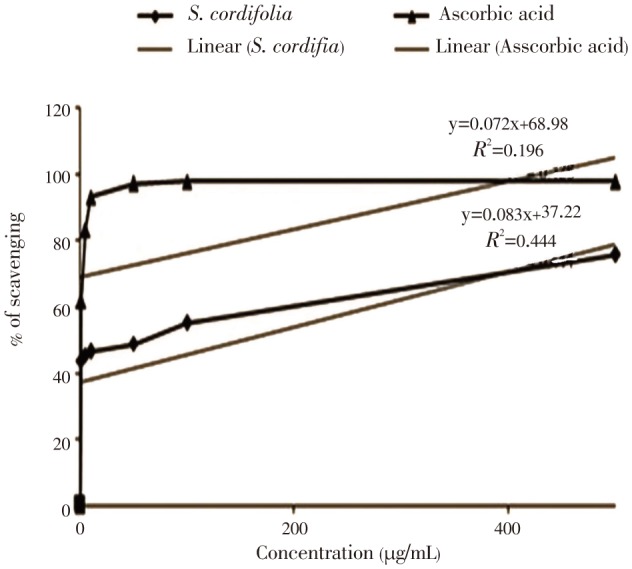
The ethanolic extract of S. cordifolia roots was tested for DPPH radical scavenging activity. The results of DPPH free radical scavenging activity on the ethanolic extracts and of ascorbic acid (standard) is shown in Figure 1, whereas the concentration of the extract increased, activity was found to increase. The IC50 value for the extract was 50 µg/mL and for ascorbic acid standard was 1.16 µg/mL.
Figure 1. DPPH radical scavenging activity of ethanolic extract of S. cordifolia.
3.3. Antimicrobial potential
Antimicrobial activities of the extract were tested against five pathogenic bacteria and compared with the standard antibiotic kanamycin by measuring the zone of inhibition diameter and expressed in mm. The results are shown in Table 2.
Table 2. Antimicrobial activity of ethanolic extract of S. cordifolia roots.
| Microorganisms | Diameter of zone of inhibition (mm) |
|
| Kanamycin (30 µg/disc) | Ethanol extract(500 µg/disc) | |
| Pseodomonas aueriginosa | 18 | 0 |
| Salmonila typhi | 18 | 0 |
| Salmenella lurea | 18 | 0 |
| Staphylococcus auerius | 18 | 0 |
| Micrococcus lutea | 18 | 0 |
The results showed the antimicrobial activity of the extracts, and it was observed that all the organisms were resistant to the extracts at 500 µg/disc concentrations. The results of antibacterial screening do not support the basis of traditional use. The experiment was conducted only with five species of bacteria, which do not at all indicate the total inactivity against micro-organisms. Therefore further researches are essential with other species of bacteria, viruses or other microorganisms.
3.4. Analgesic activity
Table 3 shows the result of statistical evaluation of the effect of ethanolic extract of S. cordifolia roots on acetic acid induced writhing in mice.
Table 3. Statistical evaluation of acetic acid induced writhing effect in mice.
| Animal group | No. of mice | Total writhing | Mean writhing | % Writhing | % Inhibition of writhing | SD | SEM | t-test (P values) |
| Control | 5 | 115 | 23.00 | 100.00 | - | 1.40 | 1.01 | - |
| Diclofenac (25 mg /kg) | 5 | 63 | 12.60 | 54.78 | 45.22 | 3.73 | 1.68 | 5.39 (P<0.001) |
| Extract (500 mg/kg) | 5 | 64 | 12.80 | 55.70 | 44.30 | 0.94 | 0.47 | 9.20 (P<0.001) |
SD: Standard deviation; SEM: Standard error of mean.
The results of the test showed that S. cordifolia ethanol extract at a dose of 500 mg/kg exhibited highly significant (P<0.001) inhibition of writhing reflex by 44.30% while the standard drug diclofenac inhibition was found to be 45.22% at a dose of 25 mg/kg body weight. The analgesic activity of the extract was significant in comparison with control animals. The extract, at dose of 500 mg/kg showed significant decrease in acetic acid induced writhing reflex of mice.
4. Discussion
A small portion of the extract was used for the phytochemical tests for compounds including reducing sugar, tannins, flavonoids, alkaloids, saponins, gums and steroids by standard method. The phytochemical analysis revealed the presence of reducing sugar, saponins, steroids and alkaloids. It has been reported that there is a linear correlation between antioxidant capacities and reducing power of plant extracts[37]. The reducing properties are generally related to the presence of reductones[38], which have been shown to exert antioxidant action by breaking the free radical chain through donating a hydrogen atom and may have great relevance in the prevention and treatment of diseases associated with oxidants or free radicals[39]. It is known that saponins produce inhibitory effect on inflammation and are the major ingredients in traditional medicine and thus responsible for most of the observed biological effects[40],[41], and this tend to justify the use of the plant in traditional medicine. The plant extract was revealed to contain saponins, so it is the justification of its traditional use. The plant extract was also positive for steroids which are very important compounds because it has a relationship with compounds such as sex hormone[42], and it has also been reported that steroids have antibacterial properties[43]. The presence of these phenolic compounds in the plant justifies the usefulness of these plants in herbal medicament and also contributed to their anti oxidative properties. Cytotoxicity is the common biological property of alkaloids and it has been associated with medicinal uses for centuries[44], and their presence in this plant tends to have the risk of poisoning by the plant. The analgesic, antispasmodic and antibacterial properties of alkaloids have also been reported by several workers[45]–[48]. The result of DPPH scavenging activity suggests that the plant extract contains compounds that are capable of donating hydrogen to a free radical in order to remove odd electron which is responsible for radical's reactivity[49]. The DPPH scavenging ability of this plant extract could also reflect its ability to inhibit the formation of ABTS+. The scavenging activity of ABTS+ radical by the extract was found to be appreciable; this implies that especially at higher concentration the plant extract may be useful for treating radical-related pathological damage[50]. It has been reported that acetic acid induced writhing method is a useful techniques for the evaluation of peripherally acting analgesic drugs[50]–[55]. So, the observed analgesic activity of the crude extract of the plant might be due to its possible interference in the biosynthesis of prostaglandins and some other autacoids. Recently many scientists have paid attention to extracts and plant origin biologically active compounds due to the side effects and resistance of the pathogenic microorganisms against antibiotics[56]. Antimicrobial activity of the plant extract was conducted against five Gram positive and Gram negative bacteria.
In conclusion, it was observed from the present study that reducing sugar, saponins, steroids and alkaloids are present in the ethanolic extract of S. cordifolia roots. These presences assume the potentiality of the antioxidant, anti-inflammatory, antibacterial and cytotoxicity activity of the plants. In DPPH scavenging test it ensured the potential antioxidant activity of the extracts which was shown by the phytochemical screening. The root extract also has the analgesic activity. The antimicrobial activity of the extract shows that all the organisms were resistant at 500 µg/disc concentrations. The experiment was conducted only with five species of bacteria as test species which do not at all indicate the total inactivity against micro-organisms. Finally it can be concluded that, further works on identification and isolation of active constituents in the extracts may be exploited by in vivo study to determine the underlying mechanism of the overall antioxidant activity. On the basis of the obtained results of antimicrobial potentials it can be suggested that, further evaluation of the antibacterial and antifungal properties of the plant extracts against a more extensive panel of microbial agents is reasonable.
Comments
Background
Plant derived medicines attracted an increasing interest since last couple of decades because of their potent pharmacological activities, convenience to users, economic viability and low toxicity. Multi-drug resistance of human pathogenic organisms to synthetic medicines enforced us to use phytomedicinal sources. This situation forced scientists to search for new antimicrobial agents from various medicinal plants like S. cordifolia. Despite the progress that has occurred in recent years in the development of therapy, there is still a need for effective and potent analgesics, especially for the treatment of chronic pain. In that sense S. cordifolia could be a good pain killer.
Research frontiers
Phytochemical analysis of the ethanol extract of the roots of S. cordifolia indicates the presence of important secondary metabolites. In vitro antioxidant (DPPH scavenging assay) the IC50 value was found 50 µg/mL which was not comparable to the standard ascorbic acid. The crude extract produced 44.30% inhibition of writhing at the dose of 500 mg/kg body weight which is statistically significant (P>0.001). In vitro antimicrobial activity of the ethanol extract of the roots of S. cordifolia showed no antimicrobial activity against Pseodomonas aueriginosa, Salmonila typhi, Salmenella lurea, Staphylococcus auerius and Micrococcus lutea.
Related reports
This study is quite different from the similar investigation reported by Franzotti et al., (2004) who searched on the anti-inflammatory, analgesic activity and acute toxicity of S. cordifolia. Islam et al., (2003) also worked on the same species of plant and the title was “Cytotoxicity and antibacterial activity of Sida rhombifolia (Malvaceae) grown in Bangladesh”. But this work is quite different from that work.
Innovations and breakthroughs
This study has explored the possibility for the use of S. cordifolia as pharmaceutical formulation without toxic effect. Use of this material against some bacterial strains which were not previously studied has been shown.
Applications
It is very significant to use this plant extract as natural medicine in bacterial infections and analgesic with appropriate doses and administration which could be studied further. This study can lead to investigating other pharmacological application of this plant.
Peer review
This is an interesting study in which the authors evaluated the analgesic, anti-inflammatory and antibacterial effects of S. cordifolia extract. Materials and methods are well designed. Findings are interesting and interpreted scientifically in discussion section.
Footnotes
Foundation Project: Supported by the Ministry of National Science, Information and Communication Technology (NSICT) of People's Republic of Bangladesh (Grant No. 12-59/04).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Rates SMK. Plants as source of drugs. Toxicon. 2001;39(5):603–613. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 2.Food and Agriculture Organization . Rome: Economic and Social Department, Food and Agriculture Organization of the United Nations; 2004. Trade in medicinal plants. [Online] Available from http://www.fao.org/docrep/008/af285e/af285e00.HTM [Accessed on 12th April, 2013] [Google Scholar]
- 3.Nair R, Kalariya T, Chanda S. Antibacterial activity of some selected Indian medicinal flora. Turk J Biol. 2005;29:41–47. [Google Scholar]
- 4.Krishnaraju BGV, Nigam SS. The in vitro antimicrobial efficiency of essential oils. Indian J Med Res. 1970;58(5):627–633. [PubMed] [Google Scholar]
- 5.Kivcak B, Mert T. Antimicrobial and cytotoxic activities of Caratonia siliqua L. extracts. Turk J Biol. 2002;26:197–200. [Google Scholar]
- 6.Ali NA, Juelich WD, Kusnick C, Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol. 2001;74(2):173–179. doi: 10.1016/s0378-8741(00)00364-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Hwang WI, Lim ST. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. J Ethnopharmacol. 2004;93(2–3):409–415. doi: 10.1016/j.jep.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Anderson D, Phillips BJ, Yu TW, Edwards AJ, Ayesh R, Butterworth KR. Effects of vitamin C supplementation in human volunteers with a range of cholesterol levels on biomarkers of oxygen radical-generated damage. Pure Appl Chem. 2000;72(6):973–983. [PubMed] [Google Scholar]
- 9.Tseng TH, Kao ES, Chu CY, Chou FP, Lin Wu HW, Wang CJ. Protective effects of dried flower extracts of Hibiscus sabdariffa L. against oxidative stress in rat primary hepatocytes. Food Chem Toxicol. 1997;35(12):1159–1164. doi: 10.1016/s0278-6915(97)85468-3. [DOI] [PubMed] [Google Scholar]
- 10.Karuna R, Sreenivasa Reddy S, Baskar R, Saralakumari D. Antioxidant potential of aqueous extract of Phyllanthus amarus in rats. Indian J Pharmacol. 2009;41(2):64–67. doi: 10.4103/0253-7613.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunin CM. Resistance to antimicrobial drugs-a worldwide calamity. Ann Intern Med. 1993;118(7):557–561. doi: 10.7326/0003-4819-118-7-199304010-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kunin CM. Antibiotic resistance-a world health problem we cannot ignore (Editorial) Ann Intern Med. 1983;99(6):859–860. doi: 10.7326/0003-4819-99-6-859. [DOI] [PubMed] [Google Scholar]
- 13.Kunin CM, Lipton HL, Tupasi T, Sacks T, Scheckler WE, Jivani A, et al. et al. Social, behavioral, and practical factors affecting antibiotic use worldwide: report of Task Force 4. Rev Infect Dis. 1987;9(Suppl 3):S270–S285. doi: 10.1093/clinids/9.supplement_3.s270. [DOI] [PubMed] [Google Scholar]
- 14.Burke JP, Levy SB. Summary report of worldwide antibiotic resistance: international task forces on antibiotic use. Rev Infect Dis. 1985;7(4):560–564. doi: 10.1093/clinids/7.4.560. [DOI] [PubMed] [Google Scholar]
- 15.Cohen ML. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992;257(5073):1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 16.Neu HC. The crisis in antibiotic resistance. Science. 1992;257(5073):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 17.Aswal BS, Bhakuni DS, Goel AK, Kar K, Mehrotra BN, Mukherjee KC. Screening of Indian plants for biological activity: Part X. Indian J Exp Biol. 1984;22(6):312–332. [PubMed] [Google Scholar]
- 18.Hoque MM, Hasan MA, Khan MR. Studies on antibacterial activity of plants available in Bangladesh 1; poligonum L. J Asiat Soc Bangladesh. 1986;12(1):77–82. [Google Scholar]
- 19.Pole K, Sebastian J. Ayurvedic medicine. Livingstone: Elsevier Health Sciences; 2006. p. 137. [Google Scholar]
- 20.Franzotti EM, Santos CVF, Rodrigues HMSL, Mourao RHV, Andrade MR, Antoniolli AR. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. J Ethnopharmacol. 2000;72(1–2):273–277. doi: 10.1016/s0378-8741(00)00205-1. [DOI] [PubMed] [Google Scholar]
- 21.Jenny M, Schwaiger W, Bernhard D, Wrulich O, Cosaceanu D, Fuchs D, et al. et al. Apoptosis induced by the Tibetan herbal remedy PADMA 28 in the T cell-derived lymphocytic leukaemia cell line CEM-C7H2. J Carcinog. 2005;4:15. doi: 10.1186/1477-3163-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam ME, Haque ME, Mossadik MA. Cytotoxicity and antibacterial activity of Sida rhombifolia (Malvaceae) grown in Bangladesh. Phytother Res. 2003;17(8):973–975. doi: 10.1002/ptr.1294. [DOI] [PubMed] [Google Scholar]
- 23.Silva RL, Melo GB, Melo VA, Antoniolli AR, Michellone PR, Zucoloto S, et al. et al. Effect of the aqueous extract of Sida cordifolia on liver regeneration after partial hepatectomy. Acta Cir Bras. 2006;21(Suppl 1):37–39. doi: 10.1590/s0102-86502006000700009. [DOI] [PubMed] [Google Scholar]
- 24.Silva RS. Effect of the aqueous extract of Sida cordifolia on liver regeneration after partial hepatectomy. Acta Cir. 2006;21(1):77–79. doi: 10.1590/s0102-86502006000700009. [DOI] [PubMed] [Google Scholar]
- 25.Gunatilaka AAL, Sotheeswaran S, Balasubramaniam S, Chandrasekara AI, Badrasriyani HT. Studies on medicinal plants of Sri Lanka. Planta Med. 1980;39:66–72. doi: 10.1055/s-2008-1074904. [DOI] [PubMed] [Google Scholar]
- 26.Franco IW, Kassulke RC, Harley KL. Host specificity and aspects of the biology of Calligrapha pantherina (COL: Chrysomelidae), a biological control agent of Sida acuta [Malvaceae] and S. rhombifola in Australia. Entomophaga. 2005;37:409–417. [Google Scholar]
- 27.Franzotti EM, Santos CV, Rodrigues HM, Mourao RH, Andrade MR, Antoniolli AR. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. J Ethnopharmacol. 2004;72(1–2):273–277. doi: 10.1016/s0378-8741(00)00205-1. [DOI] [PubMed] [Google Scholar]
- 28.Swathy SS, Panicker S, Nithya RS, Anuja MM, Rejitha S, Indira M. Antiperoxidative and antiinflammatory effect of Sida cordifolia Linn. on quinolinic acid induced neurotoxicity. Neurochem Res. 2010;35(9):1361–1367. doi: 10.1007/s11064-010-0192-5. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 30.Ghani A. Medicinal plants of Bangladesh: Chemical constituents and uses. 2nd ed. Dhaka: Asiatic Society of Bangladesh; 2003. pp. 1–16. [Google Scholar]
- 31.Trease MT, Evans SE. The phytochemical analysis and antibacterial screening of extracts of Tetracarpetum conophorum. J Chem Sci Nig. 1978;26:57–58. [Google Scholar]
- 32.Hasan MS, Ahmed MI, Mondal S, Uddin SJ, Masud MM, Sadhu SK, et al. et al. Antioxidant, antinociceptive activity and general toxicity study of Dendrophthoe falcata and isolation of quercetin as the major component. Orient Pharm Exp Med. 2006;6:355–360. [Google Scholar]
- 33.Alam MA, Nyeem MAB, Awal MA, Mostofa M, Alam MS, Subhan N, et al. et al. Antioxidant and hepatoprotective action of the crude methanolic extract of the flowering top of Rosa damascena. Orient Pharm Exp Med. 2008;8:164–170. [Google Scholar]
- 34.Bauer AW, Kirbey WMM, Sherries JC, Truck M. Antibiotic susceptibility testing by standardized single disc method. Am J Clin Pathol. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 35.Barry AL. Procedures for testing antimicrobial agents in agar media. Antibiotics in laboratory medicine. Baltimore: Willams and Wilkins Company; 1980. pp. 1–23. [Google Scholar]
- 36.Rang HP, Dale MM. Pharmacology. 2nd ed. Edinburgh: Churchill Livingstone Publisher; 1993. pp. p.706–711. [Google Scholar]
- 37.Tanaka M, Kuie CW, Nagashima Y, Taguchi T. Applications of antioxidative Maillard reaction products from histidine and glucose to sardine products. Nippon Suisan Gakkai Shi. 1988;54(8):1409–1414. [Google Scholar]
- 38.Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of harn jyur (Chrysanthemum morifolium Ramat) Lebenson Wiss Technol. 1999;32(5):269–277. [Google Scholar]
- 39.Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. London, UK: Elsevier Applied Science; 1990. pp. 1–18. [Google Scholar]
- 40.Just MJ, Recio MC, Giner RM, Cuellar MJ, Manez S, Bilia AR, et al. et al. Anti-inflammatory activity of unusual lupine saponins from Bupleurum fruticescens. Planta Med. 1998;64(5):404–407. doi: 10.1055/s-2006-957469. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Henkel T. Traditional Chineese medicine (TCM): are poly-phenols and saponins the key ingredients triggering biological activities? Curr Med Chem. 2002;9(15):1483–1485. doi: 10.2174/0929867023369709. [DOI] [PubMed] [Google Scholar]
- 42.Okwu DE. Evaluation of the chemical composition of medicinal plants belonging to Euphorbiaceae. Pak Vet J. 2001;14:160–162. [Google Scholar]
- 43.Epand RF, Savage PB, Epand RM. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins) Biochem Biophys Acta. 2007;1768(10):2500–2509. doi: 10.1016/j.bbamem.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Nobori T, Miurak K, Wu DJ, Lois A, Takabayashik LA, Carson DA. Deletion of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- 45.Harborne JB. Phytochemicals methods. London: Chapman and Hall Ltd; 1973. pp. 49–188. [Google Scholar]
- 46.Antherden LM. Textbook of pharmaceutical chemistry. 8th ed. London: Oxford University Press; 1969. pp. 813–814. [Google Scholar]
- 47.Stray F. The natural guide to medicinal herbs and plants. London: Tiger Books International; 1998. pp. 12–16. [Google Scholar]
- 48.Okwu DE, Okwu ME. Chemical composition of Spondias mombin L. plant parts. J Sustain Agric Environ. 2004;6(2):140–147. [Google Scholar]
- 49.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315(1):161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 50.Wang MF, Li JG, Rangarajan M, Shao Y, La-Voie EJ, Huang TC, et al. et al. Antioxidative phenolic compounds from sage (Salvia officinalis) J Agric Food Chem. 1998;46(12):4869–4873. [Google Scholar]
- 51.Koster R, Anderson M, De-Beer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–418. [Google Scholar]
- 52.Whittle BA. The use of changes in capillary permeability in mice to distinguish between narcotic and non-narcotic analgesics. Br J Pharmacol Chemother. 1964;22(2):246–253. doi: 10.1111/j.1476-5381.1964.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williamson EM, Okpako DT, Evans FJ. Pharmacological methods in phytotherapy research, selection, preparation and pharmacological evaluation of plant material. Volumn 1. New York: John Wiley & Sons; 1996. [Google Scholar]
- 54.Zakaria MN, Islam MW, Radhakrishnan R, Chen HB, Kamil M, Al-Gifrian A, et al. et al. Antinociceptive and anti-inflammatory properties of Caralluma arabi. J Ethnopharmacol. 2001;76(2):155–158. doi: 10.1016/s0378-8741(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 55.Silva J, Abede W, Sousa SM, Duarte VG, Machado MI, Matos FJ. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J Ethnopharmacol. 2003;89(2–3):277–283. doi: 10.1016/j.jep.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Valsaraj R, Pushpangadan P, Smitt UW, Adsersen A, Nyman U. Antimicrobial screening of selected medicinal plants from India. J Ethnopharmacol. 1997;58(2):75–83. doi: 10.1016/s0378-8741(97)00085-8. [DOI] [PubMed] [Google Scholar]


