Abstract
AIM: To investigate whether predicting patients that might be at a higher risk for complications might serve to improve the selection of patients undergoing colonic stenting.
METHODS: A retrospective review of consecutive patients who underwent an attempted self-expandable metal stent (SEMS) insertion for malignant colonic obstruction between November 2006 and March 2013. All patients were either referred for preoperative colonic decompression with the intent of a single surgical procedure, or for palliation of the malignant colorectal obstruction for unresectable cancer. Fisher’s test or χ2 test was performed on categorical variables, and the t test for continuous variables. Univariable and multivariable logistic regression were used to examine the association between independent variables and the presence of complications from SEMS insertion.
RESULTS: SEMS insertion was attempted in 73 patients. Males comprised 55.71% and the mean age was 67.41 ± 12.41 years. Of these, 65.15% underwent subsequent surgery, while 34.85% received SEMS as palliation for advanced disease. Extracolonic tumors were only 4.76%. The majority of patients had stage IV disease (63.83%), while the remainder had stage III (36.17%). SEMS were successfully inserted in 93.85% (95%CI: 87.85%-99.85%). Perforations occurred in 4.10%, SEMS migration in 8.21%, and stent re-occlusion from ingrowth occurred in 2.74% of patients. The mean duration of follow up for the patients was 13.52 ± 17.48 mo (range 0-73 mo). None of the variables: age, sex, time between the onset of symptoms to SEMS insertion, time between SEMS insertion and surgery, length of the stenosis, location of the stenosis, albumin level, or receiving neoadjuvant chemotherapy, could predict the development of complications from either SEMS insertion nor prolonged survival.
CONCLUSION: None of the variables could predict the development of complications or survival. Further studies are required to identify patients who would benefit the most from SEMS.
Keywords: Colonic obstruction, Colorectal cancer, Palliative interventions, Self-expanding metal stent, Colonic stents, Enteric stenting, Emergency surgery, Complications, Endoscopy
Core tip: Despite the debate as to whether there is an added benefit from the use of self-expandable metal stents (SEMS), when compared to surgery, as an initial management strategy in patients with malignant colorectal obstruction, this study found that SEMS insertion for malignant colonic obstruction is a safe option with an acceptable risk profile. We could not identify factors that would predict the development of complications or factors that might impact long-term survival. Nonetheless, based on current guidelines, SEMS insertion for malignant colorectal obstruction is the best option for palliation or as a bridge to surgery when technical skills for such a procedure are available.
INTRODUCTION
The use of self-expandable metal stents (SEMS) has increased in recent years, mostly either as a palliative measure or as a bridge to surgery[1-4]. There are risks associated with the use of SEMS, such as perforation[2-6], migration[2-6] and reobstruction[2-4], as well as a debate as to whether there is an added benefit from the use of SEMS when compared to surgery as an initial management strategy[7,8], and even possibly a negative effect on survival[8,9]. However, there are study design considerations that might account for such results[8,9]. As a consequence of the variability in individual study designs as well as the lack of standardization in reporting outcomes, results of numerous meta-analyses conducted on this topic have been variable[10-16]. SEMS remain an attractive option due to the avoidance of emergency surgeries, the advantage of undergoing a single operation with the avoidance of stomas[17,18], a lower early morbidity, a shorter hospital stay[11,17,18], and decreased cost[2]. Attempting to predict patients that might develop complications and identifying factors that might impact long-term survival from the insertion of SEMS for the management of malignant colorectal obstruction might aid in the better selection of patients who undergo this management strategy and who would benefit the most from SEMS.
MATERIALS AND METHODS
A retrospective review was conducted using an endoscopic reporting database of individuals seen at a major tertiary care university hospital: King Khalid University Hospital in Riyadh, Saudi Arabia. The medical records of consecutive patients who underwent an attempted SEMS insertion between November 2006 and March 2013 were included. All patients were either referred for preoperative colonic decompression with the intent of a single surgical procedure, or for palliation of the malignant colorectal obstruction of unresectable cancer. Based on a computerized tomography (CT) scan that was performed for the patients, the stage of the tumor was determined, and the SEMS insertion would be either as a bridge to surgery in patients that were deemed resectable or as a palliative procedure in those who had metastatic disease or were poor surgical candidates. All demographic features were collected through a chart review, which included: age, sex, symptoms, comorbidities, indication for SEMS insertion, date of the procedure, date of subsequent surgery (if performed), location, length of stenosis, stage of the tumor, whether the patient received neo-adjuvant chemotherapy, whether the SEMS insertion was successful (as well as the reason if it did not succeed), length of the SEMS used, number of SEMS used (if more than one SEMS was used for a patient), any complications that occurred after the SEMS insertion, duration between the initial symptoms and the SEMS insertion, and the duration between SEMS insertion and last date of follow up. Patients with any of the following were excluded: clinical evidence of bowel perforation or peritonitis, free intraperitoneal air on abdominal imaging, significant coagulopathy, hemodynamic or pulmonary instability, non-malignant strictures (e.g., those with inflammatory strictures due to diverticulitis), those where the endoscopist found a patent lumen not requiring SEMS insertion, or rectal cancer within 5 cm from the anocutaneous line.
Endoscopic technique
Before insertion of colonic SEMS, the patients underwent a CT scan of the chest, abdomen, and pelvis to evaluate the location and extent of the tumor, and to assess the area of the stenosis. SEMS were inserted by 1 of 3 therapeutic endoscopists. All endoscopies were performed under fluoroscopic guidance and were inserted through the working channel of the endoscope, which was either a therapeutic gastroscope, a colonoscope, or a duodenoscope depending on the location of the tumor and its angulation compared to the lumen of the colon. All the SEMS used were uncovered (WallFlex colonic stent), 22 mm in diameter, and 60 or 90 mm in length. The length of the stent used was dictated by the judgment of the endoscopist. The majority of the procedures were performed with the patient under conscious sedation, with intravenous midazolam and fentanyl administered by the endoscopist. Cleansing enemas were used until the washing water became clear. No oral bowel preparation was given. The endoscope was carefully inserted to the site of obstruction, then a straight tip guidewire (0.035 in diameter and 450 cm long) was inserted through a triple-lumen 5.5 French ERCP cannula through the stricture, and water soluble contrast was injected to delineate the length of the stricture, as well as the anatomy, and to confirm the intralumenal position of the guidewire. After the guidewire was passed through the stricture, a colonic SEMS assembly was advanced over the guidewire through the working channel and inserted through the obstruction site under combined fluoroscopic and endoscopic guidance. The stent was deployed at the stricture site while pulling back the outer sheath. If a SEMS did not expand, no dilation was attempted but a second stent inserted co-axially within the initial SEMS might have been used. If there was clinical suspicion of a complication, a plain abdominal radiograph was performed post-procedure. Stool softeners were routinely prescribed to prevent stool impaction in the stent. A plain abdominal radiograph the day after the procedure was performed, to confirm correct positioning and expansion of the SEMS. Successful SEMS insertion was defined as deployment and expansion of the SEMS across the stricture, radiologic and clinical relief of obstruction, and the ability to defecate. After the SEMS insertion, patients were observed for any procedure-related complications. Patients who had SEMS inserted with a palliative intent or as a bridge to surgery were followed until their last visits or death. The ethics committee of King Khalid University Hospital approved the study.
Statistical analysis
Descriptive statistics were computed for continuous variables including mean ± SD, and minimum and maximum values. Frequencies and inter-quintile ranges were used for categorical variables. Fisher’s test or χ2 test was performed on categorical variables, and the t test for continuous variables. Univariable and multivariable logistic regression were used to examine the association between independent variables and the presence of complications from SEMS insertion. OR and 95%CI were estimated. Cox proportional hazard ratio was used for survival analysis. We used the software STATA 11.2 (StataCorp, TX, United States) in our analysis. A P value of < 0.05 was considered statistically significant.
RESULTS
SEMS insertion was attempted in 73 patients. Males comprised 55.71% of the cohort and the mean age was 67.41 ± 12.41 years (95%CI: 63.50-71.33). Clinical and laboratory values for these patients are summarized in Table 1.
Table 1.
Description of the study population
| Variable | Mean | 95%CI |
| Age (yr) | 67.41 | 63.50-71.33 |
| Male | 55.71% | 43.78%-67.64% |
| Female | 44.29% | 32.36%-56.22% |
| Hemoglobin | 103 | 92-114 |
| Platelets | 273 | 238-308 |
| Creatinine | 92 | 76-109 |
| Urea | 6.76 | 4.85-8.67 |
| ALT | 37 | 30-44 |
| AST | 37 | 24-51 |
| ALP | 192 | 106-277 |
| Albumin | 30 | 29-32 |
| Total bilirubin | 17 | 8-26 |
| INR | 1.4 | 1.2-1.6 |
| CEA | 90 | 33-148 |
| Indication | ||
| Palliation of colonic tumors | 57.14% | 34.06%-80.23% |
| Complete intestinal obstruction | 38.10% | 15.44%-60.75% |
| Extracolonic tumor causing obstruction | 4.76% | 0.01%-14.70% |
| Location of the obstruction | ||
| Ascending colon | 1.45% | 0.01%-4.34% |
| Transverse colon | 4.35% | 0.01%-9.28% |
| Splenic flexure | 8.70% | 1.88%-15.51% |
| Descending colon | 7.25% | 0.97%-13.52% |
| Sigmoid colon | 69.57% | 58.44%-80.70% |
| Rectum | 8.70% | 1.88%-15.51% |
| Length of stricture (cm) | 5.16 | 4.52-5.82 |
| Stage of the tumor | ||
| Stage III | 36.17% | 21.91%-50.43% |
| Stage IV | 63.83% | 49.57%-78.09% |
| Successful SEMS insertion | 93.85% | 87.85%-99.85% |
| Failed SEMS insertion | 6.15% | 0.15%-12.15% |
| Number of SEMS inserted | ||
| A single SEMS | 87.32% | 79.39%-95.25% |
| Two SEMS | 12.68% | 4.75%-20.61% |
| Complications | ||
| Perforation | 4.10% | 0.01%-8.77% |
| Migration | 8.21% | 0.02%-14.67% |
| Stent re-occlusion | 2.74% | 0.01%-6.57% |
| Went for surgery | 65.15% | 53.35%-76.95% |
| No surgery | 34.85% | 23.05%-46.65% |
| Received neoadjuvant chemotherapy | 52.38% | 29.09%-75.68% |
| From symptom onset to SEMS insertion | 5 | 3-6 |
| From SEMS insertion to surgery | 34 | 19-49 |
| From SEMS insertion to last follow-up or death (d) | ||
| Full cohort | 425 | 297-554 |
| Patients who had surgery | 608 | 420-796 |
| Patients who had palliative therapy | 137 | 83-191 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; INR: International normalized ratio; CEA: Carcinoembryonic antigen; SEMS: Self-expandable metal stents.
Of these, 65.15% (95%CI: 53.35%-76.95%) underwent subsequent surgery while 34.85% (95%CI: 23.05%-46.65%) received SEMS for palliation for advanced disease or were not surgical candidates. The majority of the tumors were adenocarcinomas of the colon or rectum, while extracolonic tumors were only 4.76% (95%CI: 0.01%-14.70%). The obstruction in the sigmoid colon was found in 69.57%, the rectum and splenic flexure each comprising 8.70%, descending colon 7.25%, transverse colon 4.35%, and ascending colon in 1.45% (Table 1). The mean length of the strictures was 5.16 ± 0.32 cm.
Looking at time trends, there was an increased use of SEMS for malignant colorectal obstruction over the duration of the study (Figure 1). The majority (63.83%) of the patients had stage IV disease (95%CI: 49.57%-78.09%), while the remainder (36.17%) had stage III (95%CI: 21.91%-50.43%). SEMS were successfully inserted in 93.85% (95%CI: 87.85%-99.85%) of patients, while insertion failed in 6.15% (95%CI: 0.15%-12.15%). SEMS technical failure occurred in 4 patients; in 3 the guidewire could not be passed through the stricture, while in the fourth patient the SEMS would not expand. The majority of patients required one SEMS insertion 87.32% (95%CI: 79.39%-95.25%), while two SEMSs inserted in a co-axial fashion for long strictures were required in 12.68% (95%CI: 4.75%-20.61%).The mean duration from the onset of symptoms to SEMS insertion was 4.7 d, and from SEMS insertion to surgery was 33.8 d. The mean duration of follow up for the patients was 13.52 ± 17.48 mo (range 0-73 mo). Of the cohort of patients included in the study, 52.38% (95%CI: 29.09%-75.95%) received neo-adjuvant chemotherapy in our institution. Perforations occurred in 4.10%, SEMS migration in 8.21% and stent re-occlusion from ingrowth occurred in 2.74% of patients.
Figure 1.
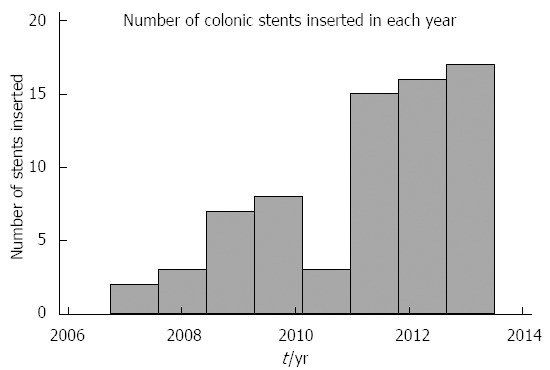
The number of colonic stents inserted for malignant colonic obstruction over the study period.
Predictors of complications from SEMS insertion:
On hypothesis testing, there was no association between any of the measured variables and the development of complications (Table 2). On univariable analysis, none of the following variables predicted the development of complications (perforation, migration, and stent re-occlusion) from SEMS insertion: patient age (OR = 1.02, 95%CI: 0.95-1.10), patient gender (OR = 2.37, 95%CI: 0.69-8.14), time between the onset of symptoms to SEMS insertion (OR = 1.01, 95%CI: 0.99-1.03), time between SEMS insertion and surgery (OR =1.02, 95%CI: 0.85-1.22), length of the stenosis (OR = 1.12, 95%CI: 0.70-1.80), location of the stenosis (OR = 1.03, 95%CI: 0.97-1.08), albumin level (OR = 0.98, 95%CI: 0.90-1.06), or receiving neoadjuvant chemotherapy (OR = 1.38, 95%CI: 0.39-4.88). Also, on multivariable analysis, none of the variables were associated with the development of complications from SEMS insertion in malignant colorectal obstruction.
Table 2.
Factors associated with the development of complications (perforation, migration, and stent re-occlusion)
| Variables |
Complication |
P value | |||
|
Yes |
No |
||||
| Mean | 95%CI | Mean | 95%CI | ||
| Age | 70 | 58.37-81.63 | 66.97 | 62.60- 71.34 | 0.56 |
| Sex | |||||
| Male | 12.82% | 2.00%-23.64% | 87.18% | 76.36%-98.00% | 0.17 |
| Female | 25.80% | 9.87%-41.74% | 74.19% | 58.26%-90.13% | |
| Hemoglobin | 106 | 73-140 | 102 | 92-114 | 0.84 |
| Platelet count | 287 | 176-398 | 271 | 234-308 | 0.38 |
| Creatinine | 90 | 76-104 | 93 | 73-113 | 0.76 |
| Urea | 5.44 | 2.78-8.09 | 7.04 | 4.77-9.32 | 0.83 |
| Albumin | 29.31 | 25.11-33.50 | 30.49 | 28.56-32.43 | 0.59 |
| Alanine aminotransferase | 53 | 17-88 | 34 | 30-37 | 0.28 |
| Aspartate aminotransferase | 86 | 7-165 | 28 | 21-34 | 0.13 |
| Alkaline phosphatase | 439 | 1-930 | 144 | 92-196 | 0.21 |
| Total bilirubin | 37 | 1-88 | 13 | 8-17 | 0.33 |
| International normalized ratio | 1.15 | 1.06-1.24 | 1.44 | 1.19-1.68 | 0.03 |
| Carcinoembryonic antigen | 188 | 0-439 | 69 | 19-148 | 0.32 |
| Length of stenosis (cm) | 5.43 | 4.75-6.11 | 5.09 | 4.24-5.93 | 0.49 |
| Duration between symptoms and stenting (d) | 5 | 1.62-8.38 | 4.59 | 2.94-6.24 | 0.80 |
| Duration between stenting and surgery (d) | 51.33 | 10.96-91.70 | 28.34 | 11.76-44.93 | 0.26 |
| Neo-adjuvant chemotherapy | 17.14% | 4.22%-30.07% | 22.22% | 5.92%-38.53% | 0.62 |
| Number of stents | 1.15 | 0.93-1.38 | 1.1 | 1.03-1.20 | 0.66 |
Predictors of survival
There was a difference in survival between the patients receiving SEMS as a palliative therapy (4.1 ± 3.08 mo) and those who had SEMS inserted as a bridge to surgery (19.4 ± 0.83 mo) (Figure 1). We think that this is a function of the stage of the disease; stage III with a mean duration of 21.88 ± 5.98 mo vs stage IV with a mean duration of follow-up of 7.36 ± 1.93 mo (Figure 2A). On univariable analysis, the albumin level was associated with a survival advantage (P < 0.01). None of the following predicted long term survival: time between the onset of symptoms to SEMS insertion (P = 0.91), time between SEMS insertion and surgery (P = 0.44), location of the stenosis (P = 0.43), length of the stenosis (P = 0.95), development of complications from SEMS insertion (P = 0.07), carcinoembryonic antigen level (P = 0.10), or neoadjuvant chemotherapy (P = 0.71). On multivariate analysis, none of the variables were associated with long-term survival.
Figure 2.
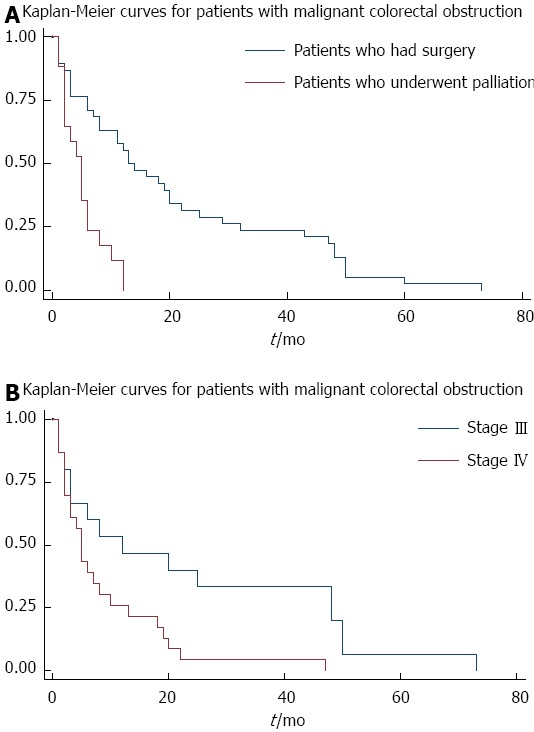
Survival curves. A: Survival curves for patients who received self-expandable metal stents for malignant colorectal obstruction stratified by those who had surgery and those who had palliation; B: Survival curves for patients who received self-expandable metal stents for malignant colorectal obstruction that stratified the stage of their disease.
DISCUSSION
SEMS are a reasonably safe option for patients with malignant colorectal obstruction[19]. The aim for the insertion of SEMS includes decreasing the need for emergency surgeries, reducing the rate of stomas, facilitation of laparoscopic resection when surgery is indicated, decreasing morbidity, shortening the time to chemotherapy, improving the quality of life for patients, and being more cost-effective[3,15-21]. Despite these potential and important endpoints and numerous studies addressing the use of SEMS for malignant colorectal obstruction, there still remains considerable controversy concerning their added benefit when compared to surgery as a dominant strategy for managing these patients[5,7,20,22]. This is mostly due to methodological issues in these studies that are inherited in their design, as well as the possibility of selection bias, being underpowered in detecting differences between study arms, lack of standardized outcomes (as well as definitions), heterogeneity of the patients included, underlying origin of the tumor, stage of the disease, and the use of covered or uncovered SEMS[20].
In a meta-analysis that included 601 patients, of which 38.6% underwent colonic SEMS insertion compared to emergency surgery, the SEMS group had a reduced risk of requiring intensive care with a risk ratio (RR = 0.42, 95%CI: 0.19-0.93), the need for a stoma (RR = 0.70, 95%CI: 0.50-0.99), reduced anastomotic leakage (RR = 0.31 (95%CI: 0.14-0.69), and reduced complications (RR = 0.42, 95%CI: 0.24-0.71)[15]. Furthermore, SEMS insertion prior to surgery did not affect the mortality or long-term survival[15]. Although encouraging, the meta-analysis by Zhang et al[15] had considerable heterogeneity, and included various study designs between observational and randomized studies[20]. In an editorial by Dayyeh et al[20], a meta-analysis was attempted and included only randomized trials[23-26]. It demonstrated that there was a decrease in the rate of stomas with the use of SEMS for malignant colorectal obstruction (RR = 0.68, 95%CI: 0.53-0.89), and no difference in complications when compared to emergent surgery (RR = 0.88, 95%CI: 0.66-1.18)[20]. The authors correctly pointed out that when examining studies with a high technical success rate in inserting the SEMS, they had a more favorable complication profile when compared to emergent surgery[20], and that possibly the focus should be on the probability of inserting a SEMS in patients with malignant colorectal obstruction, as a determinant for using it as a management strategy[4,20].
The benefits of SEMS for right-sided malignant colorectal obstruction are less than that of distal lesions, as right hemicolectomy with primary anastomosis is possible even in an unprepared colon, although it avoids emergent surgery and possibly permits preoperative medical optimization of patients[4].
Our cohort had a mean age similar to other studies[1,3,23,27], with about half the study population being female, which is higher than in some series[1,27]. Also, in other series, 27%-41% of patients who underwent colonic SEMS insertion with a palliative intent had extracolonic tumor origins[1,27,28], while only 4.8% of our series had extracolonic tumors, which is similar to the cohort by Jiménez-Pérez et al[3]. These salient differences in patient characteristics can explain some of the variation in study results, as patients who had SEMS inserted for malignant colonic obstruction from extraintestinal tumor origins were more likely to be unsuccessful[6,29], but in those receiving SEMS with a palliative intent there was no difference in the SEMS patency and reobstruction rate (21.9% vs 30%, P = 0.29)[28].
The location of the obstruction was mostly in the sigmoid colon and the majority was on the left side of the colon, this is also in keeping with the literature[1,3,27,28].
The rate of patients with complete obstruction in this cohort (38.1%) was lower than that by Yoon et al[1] (73%), but a number of series have included patients with incomplete obstruction, defined as a state with narrow stool caliber or the ability to pass only small amounts of liquid stool or gas. We had a lower rate of patients with stage IV (64%) disease when compared to others (92%)[27], although it was still similar to some series[30]. One of the concerns with some of the series, and which limits the generalization of their results, is a lack in reporting the stage of the disease; a known independent factor affecting the overall survival of patients.
The success rate for SEMS insertion in our cohort was 93.85%. This is similar to that reported in the literature (83%-100%)[27,28,31]. Our study exclusively used uncovered SEMS, since covered SEMS had no added benefit when compared to uncovered SEMS with regards to technical or clinical success. Covered SEMS had a higher proportion of late migration (40% vs 0%) and loss of function during the long-term follow-up (60% vs 18.8%)[32]. A randomized trial also demonstrated that although there was a higher rate of ingrowth when using uncovered SEMS (14.5% vs 3.8%), the rate of migration was higher in the covered SEMS group (21.1% vs 1.8%), with no difference in the mean patency rate[33]. The migration rate in this study was 8.21%; this is in keeping with that reported by others (1%-6%)[3,27], as well as a pooled analysis that found the migration rate to be 11.81%[34]. Kim et al[30] found that covered SEMS and those with a diameter of less than 24 mm had a higher risk of migration. The perforation rate was 4.1%. A pooled analysis of 2287 patients found the perforation rate to be 4.9%, with no statistical difference between the use of stents as a bridge to surgery or palliation[34]. The rate of silent perforations could not be assessed in our study, although it was been reported to be as high as 20% in a randomized trial[23].
In our series, none of the patient or tumor characteristics were a predictor for complications from SEMS insertion. This may well be due to the low number of events in this cohort. A study by Kim et al[28] did not find any predictors for failed SEMS insertion, while in a series of 412 patients, Yoon et al[1] described predictors for technical and clinical failure in patients who underwent SEMS insertion. Factors associated with technical failure were: right-sided obstruction (OR = 2.25, 95%CI: 1.06-4.75), extrinsic origin of malignancy (OR = 2.57, 95%CI: 1.25-5.32), and the presence of carcinomatosis (OR = 2.83, 95%CI: 1.19-6.75). Factors associated with long term clinical failure, defined as the recurrence of obstructive symptoms requiring re-intervention after initial relief, were when balloon dilatation was used in combination with SEMS insertion (OR = 3.58, 95%CI: 1.25-5.32), while it was decreased when the patient received additional chemotherapy (OR = 0.52, 95%CI: 0.31-0.88)[1].
Since we did not have the exact mortality data, we conducted the survival analysis until the patients’ death, when known, or till the last date of follow up. The mean duration of follow up for the complete cohort was 425 d (95%CI: 297-554). As expected, the patients receiving subsequent surgery after SEMS insertion had a longer survival than those who had SEMS inserted with a palliative intent; 608 d vs 137 d respectively (Figure 2A). The authors think this is mainly due to the stage of the disease (Figure 2B), and also probably due to unmeasured factors like the functional status of the patients. On univariable analysis, the albumin level was found to be associated with a better survival, but this probably reflected the overall health of the patient. Thus, on multivariable analysis, this variable was not associated with a survival advantage. Jung et al[35] found that the location of the tumor affected the mean event-free survival, with distal obstructions being better than proximal lesions; 122.9 ± 18.6 d vs 35.8 ± 12.8 d respectively[35]. Additionally, SEMS < 10 cm long had a better mean event-free survival when compared to those > 10 cm; 151.0 ± 24.5 d vs 59.5 ± 14.4 d respectively[35]. The median duration of stent patency in patients with malignant colorectal obstruction was 193 ± 42 - 200 d[27,28]. This was not affected by patient demographics[27], site of obstruction[27], or the administration of palliative chemotherapy[27,28].
We also demonstrated through this study that side-viewing duodenoscopes can be used successfully (as was the case in at least three patients in this series) for better visualizing of the tumor and targeting the insertion of a guidewire in areas where the tumor is situated in a tight angle in the distal colon[4,36].
Guidelines on the management of left-sided colonic obstructions state that, in facilities where SEMS insertion is possible, they should be preferred to colostomy, since SEMS have a similar mortality/morbidity rate and a shorter hospital stay (grade of recommendation 2B)[37]. The guidelines also suggested considering alternative treatments to SEMS in patients eligible for further bevacizumab-based therapy, due to the potentially increased perforation rates[37]. Furthermore, the guidelines state that SEMS should be used as a bridge to elective surgery in referral centers with specific expertise and in selected patients, as their use seems to be associated with a lower mortality rate, a shorter hospital stay, and a lower colostomy rate (grade of recommendation 1B)[37].
In conclusion, none of the variables in our study could predict the occurrence of complications (perforation, migration, and stent re-occlusion) from the insertion of SEMS or long-term survival in cases with malignant colonic obstruction. This may well be due to the size of the cohort in this study. Based on current guidelines[37], as well as in a technical review[4], SEMS insertion for malignant colorectal obstruction is the best option for palliation or as a bridge to surgery when technical skills for such a procedure are available[4].
COMMENTS
Background
In patients presenting with malignant colorectal obstruction there is a debate in the literature about the best management strategy that would translate to decreased morbidity, mortality, and cost to the health care system. Despite some randomized controlled trials on the use of self-expandable metal stents (SEMS) or surgery, the answer is not clear due to the variability in the results, as well as the wide variability in the frequency of adverse events from the use of SEMS.
Research frontiers
SEMS are an attractive management strategy for the management of malignant colorectal obstruction, as it allows for the avoidance of emergency surgeries as providing a single operation without the need for stomas, lower early morbidity, a shorter hospital stay, and decreased costs when compared to emergency surgery. In this study, the authors have demonstrated that the insertion of SEMS in cases with malignant colorectal obstruction is effective with an acceptable risk profile, but could not find any predictors that could determine the development of complications or survival.
Innovations and breakthroughs
Despite the conduction of randomized controlled trials with regards to an emergent surgery strategy compared to the insertion of SEMS as a bridge to surgery, there are considerable arguments with regards to the outcomes and the proportion of complications encountered during the insertion of SEMS. This study, although retrospective, replicates that the insertion of SEMS is relatively safe and the rate of complications is less than that reported in some randomized trials.
Applications
By identifying factors that might predict complications or a survival advantage from one treatment modality compared to another, the authors could individually tailor the best management strategy for patients who present with malignant colorectal obstruction.
Terminology
SEMS: tubes that are made of a metallic material and are inserted into the colon through the use of endoscopes. These are usually deployed in individuals who have developed a blockage of the colon, most commonly due to malignancy. Migration: when the stent has moved from its intended position to an area either before or after the area of obstruction. Ingrowth: when the tumor tissue extends through the mesh network of the stent and causes occlusion of the stent.
Peer review
This is a well-written manuscript on an important topic. The authors aimed to predict complications after stent placement for colonic obstruction either in a palliative or a curative attempt. The purpose of the study is interesting and could help in selecting patients who present with malignant colorectal obstruction and would be good candidates for the insertion of SEMS.
Footnotes
Supported by The Deanship of Scientific Research at King Saud University funded this research through the Research Group Project, No. RGP-VPP-279
P- Reviewers: Catena F, Gupta C, Il Kim T, Regimbeau JM S- Editor: Zhai HH L- Editor: Rutherford A E- Editor: Ma S
References
- 1.Yoon JY, Jung YS, Hong SP, Kim TI, Kim WH, Cheon JH. Clinical outcomes and risk factors for technical and clinical failures of self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointest Endosc. 2011;74:858–868. doi: 10.1016/j.gie.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 2.Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051–2057. doi: 10.1111/j.1572-0241.2004.40017.x. [DOI] [PubMed] [Google Scholar]
- 3.Jiménez-Pérez J, Casellas J, García-Cano J, Vandervoort J, García-Escribano OR, Barcenilla J, Delgado AA, Goldberg P, Gonzalez-Huix F, Vázquez-Astray E, et al. Colonic stenting as a bridge to surgery in malignant large-bowel obstruction: a report from two large multinational registries. Am J Gastroenterol. 2011;106:2174–2180. doi: 10.1038/ajg.2011.360. [DOI] [PubMed] [Google Scholar]
- 4.Baron TH, Wong Kee Song LM, Repici A. Role of self-expandable stents for patients with colon cancer (with videos) Gastrointest Endosc. 2012;75:653–662. doi: 10.1016/j.gie.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 5.van Hooft JE, Fockens P, Marinelli AW, Timmer R, van Berkel AM, Bossuyt PM, Bemelman WA. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40:184–191. doi: 10.1055/s-2007-995426. [DOI] [PubMed] [Google Scholar]
- 6.Keränen I, Lepistö A, Udd M, Halttunen J, Kylänpää L. Stenting for malignant colorectal obstruction: a single-center experience with 101 patients. Surg Endosc. 2012;26:423–430. doi: 10.1007/s00464-011-1890-z. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Esparrach G, Bordas JM, Giráldez MD, Ginès A, Pellisé M, Sendino O, Martínez-Pallí G, Castells A, Llach J. Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol. 2010;105:1087–1093. doi: 10.1038/ajg.2009.660. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Hur H, Min BS, Sohn SK, Cho CH, Kim NK. Oncologic outcomes of self-expanding metallic stent insertion as a bridge to surgery in the management of left-sided colon cancer obstruction: comparison with nonobstructing elective surgery. World J Surg. 2009;33:1281–1286. doi: 10.1007/s00268-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 9.Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, Mauvais F, Chauffert B, Dupas JL, Nguyen-Khac E, et al. Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg. 2013;258:107–115. doi: 10.1097/SLA.0b013e31827e30ce. [DOI] [PubMed] [Google Scholar]
- 10.Cennamo V, Luigiano C, Coccolini F, Fabbri C, Bassi M, De Caro G, Ceroni L, Maimone A, Ravelli P, Ansaloni L. Meta-analysis of randomized trials comparing endoscopic stenting and surgical decompression for colorectal cancer obstruction. Int J Colorectal Dis. 2013;28:855–863. doi: 10.1007/s00384-012-1599-z. [DOI] [PubMed] [Google Scholar]
- 11.Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev. 2011;(11):CD007378. doi: 10.1002/14651858.CD007378.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan CJ, Dasari BV, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg. 2012;99:469–476. doi: 10.1002/bjs.8689. [DOI] [PubMed] [Google Scholar]
- 13.Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013;22:14–21. doi: 10.1016/j.suronc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Ye GY, Cui Z, Chen L, Zhong M. Colonic stenting vs emergent surgery for acute left-sided malignant colonic obstruction: a systematic review and meta-analysis. World J Gastroenterol. 2012;18:5608–5615. doi: 10.3748/wjg.v18.i39.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc. 2012;26:110–119. doi: 10.1007/s00464-011-1835-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Comparison of efficacy between uncovered and covered self-expanding metallic stents in malignant large bowel obstruction: a systematic review and meta-analysis. Colorectal Dis. 2012;14:e367–e374. doi: 10.1111/j.1463-1318.2012.03056.x. [DOI] [PubMed] [Google Scholar]
- 17.Súarez J, Jiménez J, Vera R, Tarifa A, Balén E, Arrazubi V, Vila J, Lera JM. Stent or surgery for incurable obstructive colorectal cancer: an individualized decision. Int J Colorectal Dis. 2010;25:91–96. doi: 10.1007/s00384-009-0814-z. [DOI] [PubMed] [Google Scholar]
- 18.Lee WS, Baek JH, Kang JM, Choi S, Kwon KA. The outcome after stent placement or surgery as the initial treatment for obstructive primary tumor in patients with stage IV colon cancer. Am J Surg. 2012;203:715–719. doi: 10.1016/j.amjsurg.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Donnellan F, Cullen G, Cagney D, O’Halloran P, Harewood GC, Murray FE, Patchett SE. Efficacy and safety of colonic stenting for malignant disease in the elderly. Int J Colorectal Dis. 2010;25:747–750. doi: 10.1007/s00384-010-0917-6. [DOI] [PubMed] [Google Scholar]
- 20.Dayyeh BK, Baron TH. Editorial: endoscopic stent placement as a bridge to surgery in malignant colorectal obstruction: a balance between study validity and real-world applicability. Am J Gastroenterol. 2011;106:2181–2182. doi: 10.1038/ajg.2011.362. [DOI] [PubMed] [Google Scholar]
- 21.Govindarajan A, Naimark D, Coburn NG, Smith AJ, Law CH. Use of colonic stents in emergent malignant left colonic obstruction: a Markov chain Monte Carlo decision analysis. Dis Colon Rectum. 2007;50:1811–1824. doi: 10.1007/s10350-007-9047-9. [DOI] [PubMed] [Google Scholar]
- 22.Grundmann RT. Primary colon resection or Hartmann’s procedure in malignant left-sided large bowel obstruction? The use of stents as a bridge to surgery. World J Gastrointest Surg. 2013;5:1–4. doi: 10.4240/wjgs.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12:344–352. doi: 10.1016/S1470-2045(11)70035-3. [DOI] [PubMed] [Google Scholar]
- 24.Cheung HY, Chung CC, Tsang WW, Wong JC, Yau KK, Li MK. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg. 2009;144:1127–1132. doi: 10.1001/archsurg.2009.216. [DOI] [PubMed] [Google Scholar]
- 25.Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc. 2011;25:1814–1821. doi: 10.1007/s00464-010-1471-6. [DOI] [PubMed] [Google Scholar]
- 26.Alcántara M, Serra-Aracil X, Falcó J, Mora L, Bombardó J, Navarro S. Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg. 2011;35:1904–1910. doi: 10.1007/s00268-011-1139-y. [DOI] [PubMed] [Google Scholar]
- 27.Im JP, Kim SG, Kang HW, Kim JS, Jung HC, Song IS. Clinical outcomes and patency of self-expanding metal stents in patients with malignant colorectal obstruction: a prospective single center study. Int J Colorectal Dis. 2008;23:789–794. doi: 10.1007/s00384-008-0477-1. [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Kim SG, Im JP, Kim JS, Jung HC. Comparison of treatment outcomes of endoscopic stenting for colonic and extracolonic malignant obstruction. Surg Endosc. 2013;27:272–277. doi: 10.1007/s00464-012-2439-5. [DOI] [PubMed] [Google Scholar]
- 29.Keswani RN, Azar RR, Edmundowicz SA, Zhang Q, Ammar T, Banerjee B, Early DS, Jonnalagadda SS. Stenting for malignant colonic obstruction: a comparison of efficacy and complications in colonic versus extracolonic malignancy. Gastrointest Endosc. 2009;69:675–680. doi: 10.1016/j.gie.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Kim BC, Han KS, Hong CW, Sohn DK, Park JW, Park SC, Kim SY, Baek JY, Choi HS, Chang HJ, et al. Clinical outcomes of palliative self-expanding metallic stents in patients with malignant colorectal obstruction. J Dig Dis. 2012;13:258–266. doi: 10.1111/j.1751-2980.2012.00564.x. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh DO, Nolan B, Judge C, Hyland JM, Mulcahy HE, O’Connell PR, Winter DC, Doherty GA. A comparative study of short- and medium-term outcomes comparing emergent surgery and stenting as a bridge to surgery in patients with acute malignant colonic obstruction. Dis Colon Rectum. 2013;56:433–440. doi: 10.1097/DCR.0b013e3182760506. [DOI] [PubMed] [Google Scholar]
- 32.Lee KM, Shin SJ, Hwang JC, Cheong JY, Yoo BM, Lee KJ, Hahm KB, Kim JH, Cho SW. Comparison of uncovered stent with covered stent for treatment of malignant colorectal obstruction. Gastrointest Endosc. 2007;66:931–936. doi: 10.1016/j.gie.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Cheon JH, Park JJ, Moon CM, Hong SP, Lee SK, Kim TI, Kim WH. Comparison of efficacies between stents for malignant colorectal obstruction: a randomized, prospective study. Gastrointest Endosc. 2010;72:304–310. doi: 10.1016/j.gie.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 34.Datye A, Hersh J. Colonic perforation after stent placement for malignant colorectal obstruction--causes and contributing factors. Minim Invasive Ther Allied Technol. 2011;20:133–140. doi: 10.3109/13645706.2010.518787. [DOI] [PubMed] [Google Scholar]
- 35.Jung MK, Park SY, Jeon SW, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Kim GC, Ryeom HK. Factors associated with the long-term outcome of a self-expandable colon stent used for palliation of malignant colorectal obstruction. Surg Endosc. 2010;24:525–530. doi: 10.1007/s00464-009-0604-2. [DOI] [PubMed] [Google Scholar]
- 36.Cennamo V, Fuccio L, Laterza L, Ceroni L, Eusebi LH, Fabbri C, Bazzoli F. Side-viewing endoscope for colonic self-expandable metal stenting in patients with malignant colonic obstruction. Eur J Gastroenterol Hepatol. 2009;21:585–586. doi: 10.1097/MEG.0b013e328318ed54. [DOI] [PubMed] [Google Scholar]
- 37.Ansaloni L, Andersson RE, Bazzoli F, Catena F, Cennamo V, Di Saverio S, Fuccio L, Jeekel H, Leppäniemi A, Moore E, et al. Guidelenines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J Emerg Surg. 2010;5:29. doi: 10.1186/1749-7922-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]


