Abstract
Limited treatment options exist for isolated local recurrence of pancreatic ductal adenocarcinoma (PDA) following surgical resection accompanied by neoadjuvant or adjuvant chemoradiation therapy (CRT). While select patients are eligible for re-resection, recurrent lesions are often unresectable. Stereotactic body radiation therapy (SBRT) represents a possible minimally invasive treatment option for these patients, although published data in this setting are currently lacking. This study examines the safety, efficacy, and palliative capacity of re-irradiation with SBRT for isolated local PDA recurrence.
All patients undergoing SBRT at two academic centers from 2008-2012 were retrospectively reviewed to identify those who received re-irradiation with SBRT for isolated local recurrence or progression of PDA after previous conventionally fractionated CRT. Information regarding demographics, clinicopathologic characteristics, therapies received, survival, symptom palliation, and toxicity was obtained from patient charts. Kaplan-Meier statistics were used to analyze survival and the log-rank test was used to compare survival among patient subgroups.
Eighteen patients were identified. Fifteen had previously undergone resection with neoadjuvant or adjuvant CRT, while 3 received definitive CRT for locally advanced disease. Median CRT dose was 50.4 Gy [interquartile range (IQR), 45.0-50.4 Gy] in 28 fractions. All patients subsequently received gemcitabine-based maintenance chemotherapy, but developed isolated local disease recurrence or progression without evidence of distant metastasis. Locally recurrent or progressive disease was treated with SBRT to a median dose of 25.0 Gy (range, 20.0-27.0 Gy) in 5 fractions. Median survival from SBRT was 8.8 months (95% CI, 1.2-16.4 months). Despite having similar clinicopathologic disease characteristics, patients who experienced local progression greater than vs. less than 9 months after surgery/definitive CRT demonstrated superior median survival (11.3 vs. 3.4 months; P=0.019) and progression-free survival (10.6 vs. 3.2 months; P=0.030) after SBRT. Rates of freedom from local progression at 6 and 12 months after SBRT were 78% (14 of 18 patients) and 62% (5 of 8 patients), respectively. Effective symptom palliation was achieved in 4 of 7 patients (57%) who reported symptoms of abdominal or back pain prior to SBRT. Five patients (28%) experienced grade 2 acute toxicity; none experienced grade ≥3 acute toxicity. One patient (6%) experienced grade 3 late toxicity in the form of small bowel obstruction.
In conclusion, re-irradiation with hypofractionated SBRT in this salvage scenario appears to be a safe and reasonable option for palliation of isolated local PDA recurrence or progression following previous conventional CRT. Patients with a progression-free interval of greater than 9 months prior to isolated local recurrence or progression may be most suitable for re-irradiation with SBRT, as they appear to have a better prognosis with survival that is long enough for local control to be of potential benefit.
Key Words: Stereotactic body radiation therapy (SBRT), pancreatic cancer, local recurrence, re-irradiation
Introduction
Recurrent pancreatic cancer after definitive treatment with multimodality therapy universally portends a dismal prognosis, with 5-year survival rates of 5.6% or less (1,2). Unfortunately, this scenario is not uncommon. Among the minority of patients (10-15%) able to undergo potentially curative surgical resection, more than 80% subsequently recur (3,4).
The pattern of recurrence in pancreatic cancer is well-known (4-10). Following resection, approximately 70% develop distant metastases within 2 years, often accompanied by synchronous local recurrence (11,12), while up to 30% exhibit isolated local recurrence (10,13). Autopsy studies have demonstrated that 30% of deaths are due to locally progressive disease, while the remainder result from distant metastases (14). Symptomatic manifestations of local recurrence include pain, bowel obstruction, portal hypertension, biliary obstruction, and malnutrition (15). Although survival is determined chiefly by systemic progression, local progression is an important factor contributing to quality of life (16) and has been associated with decreased time to metastasis (16).
Current therapeutic approaches for patients who develop isolated local recurrence following conventionally-fractionated radiotherapy include palliative chemotherapy and best supportive care, with a very select few undergoing surgical re-resection. Each of these options has significant drawbacks, including: invasiveness and morbidity with re-resection (2,17); systemic toxicity and modest local control with palliative chemotherapy (18); and lack of efficacy with supportive care alone. A possible alternative in this salvage scenario is re-irradiation with stereotactic body radiation therapy (SBRT). SBRT is minimally invasive, can be administered over 5 days or fewer, and may offer a high probability of local control (19-23). Herein, we present a retrospective study of re-irradiation using SBRT at two institutions.
Methods
Patient selection
With IRB approval, records of all pancreatic cancer patients treated with SBRT at two academic centers from 2008-2012 were retrospectively reviewed to identify patients with isolated local recurrence (if previously resected) or isolated local progression (if locally advanced disease) after previous conventional radiotherapy and who subsequently received salvage SBRT. Patients were required to meet the following inclusion criteria: age ≥18, Eastern Cooperative Oncology Group (ECOG) performance status 0-2, histologically confirmed pancreatic adenocarcinoma, local disease recurrence/progression determined by a radiologist on pancreas-protocol CT scan following conventionally-fractionated radiotherapy (≤300 cGy/fraction), and salvage SBRT. Patients with radiographic evidence of distant metastasis prior to SBRT were excluded. Patients received heterogeneous systemic therapies before and/or following re-irradiation with SBRT, but no other forms of local therapy. No exclusions were made based on systemic therapies received.
SBRT planning
Patients underwent simulation supine in an immobilization device. An arterial-phase pancreas-protocol CT scan (1.25-mm slices from T4/T5-L5/S1) with IV and oral contrast was obtained during expiration breath-hold for tumor delineation. A free-breathing CT scan with 4-D respiratory correlation was also obtained to characterize target motion during quiet respiration. If target motion was >5 mm, respiratory gating using the Varian Respiratory Management System™ (Stanford), Cyberknife™ respiratory tracking (Stanford), or the Elekta Active Breathing Coordinator System™ (Hopkins) was utilized during treatment delivery. When available (12 of 18 patients), FDG-PET/CT scans were fused with simulation scans.
SBRT treatment plans were developed using Eclipse™ (Varian, Palo Alto, CA), Multi-Plan™ (Accuray, Sunnyvale, CA), or Pinnacle™ (Philips, Amsterdam, Netherlands). The gross tumor volume (GTV) was contoured by a radiation oncologist using the simulation scan. An internal target volume (ITV) was then defined after review of diagnostic imaging, respiratory-correlated 4D-CT, pancreas-protocol CT, and FDG-PET/CT scans. Final planning target volume (PTV) was obtained by an additional 1-3 mm uniform margin expansion of the ITV. The dose was prescribed to the isodose line that completely surrounded the PTV and 6-12 co-planar fields were used to generate the plan for non-Cyberknife™ treatments. Dose constraints for organs at risk were employed as follows: duodenum—V15Gy<9 cc, V20Gy<3 cc, V33Gy<1 cc; liver—D50%<12 Gy; stomach—D50%<12 Gy, V33Gy<1 cc; spinal cord—V8Gy<1 cc. Institutional standards for patient-specific dosimetric quality assurance were applied.
SBRT delivery
For non-Cyberknife™-based treatment (N=11), initial patient position was based on cone-beam CT with alignment to spine. Volumetric kV-imaging was then used to align biliary stent and/or fiducials to the digitally-reconstructed radiograph. All fiducials were placed specifically for SBRT image guidance using an endoscopic approach (N=11 patients); complications of fiducial placement were observed in only one patient who experienced laryngospasm and had to return for repeat endoscopy the following day. Common bile duct stents were placed endoscopically for relief of symptomatic biliary obstruction and not for purposes of SBRT image guidance, but if a stent was present, then fiducial placement was deemed unnecessary (N=4 patients). If a stent or fiducials were not present, patients were aligned to spine only (N=3). In patients who had previously undergone intra-tumoral fiducial placement, orthogonal kV/MV or kV/kV projection imaging was used to verify fiducial location before first treatment beam delivery and, if indicated, a secondary shift was performed. Active monitoring of treatment delivery accuracy was accomplished using kV and MV projection imaging.
For CyberKnife™-based treatment (N=7; fiducials required), initial orthogonal kV/MV or kV/kV projection images were obtained to confirm fiducial location. The Synchrony™ respiratory tracking system (Accuray) was then used to correct for tumor-associated motion using a series of optical diodes placed on the patient’s chest wall and correlated to the internal fiducials by a computer to generate a model continuously updated during treatment to correct for subtle changes in tumor location.
Clinical outcomes
Clinical data were gathered by retrospective chart review using electronic patient records. Date of progression was defined as the date of first follow-up cross-sectional imaging study showing evidence of distant metastases or local progression as determined by an attending radiologist. Survival was measured from the date of the first fraction of SBRT until date of death or censored at the date of last follow-up if no date of death was available. Toxicity was evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistics
Patient demographic, clinicopathologic, and treatment characteristics were summarized using descriptive statistics. Patient characteristics were compared among different patient subgroups using the Mann-Whitney U test for comparison of medians and the Pearson chi-square test for comparison of proportions. Survival data were analyzed using Kaplan-Meier statistics and compared between subgroups using the log-rank test. A two-sided alpha level of ≤0.05 was considered significant in all cases. Statistical analyses were performed with SPSS (IBM, Armonk, NY).
Results
Patients
Eighteen patients were identified. Complete demographic, baseline, and treatment characteristics are summarized in Table 1. Fifteen patients received neoadjuvant or adjuvant CRT in association with surgical resection, while 3 received definitive CRT for locally advanced disease. Median CRT dose was 50.4 Gy (IQR, 45.0-50.4 Gy) in 28 fractions with median daily fraction size of 1.8 Gy (IQR, 1.8-1.8 Gy). Seventeen of 18 patients (94%) received chemotherapy concurrently with radiotherapy. All patients subsequently received gemcitabine-based maintenance chemotherapy, but eventually developed isolated local disease recurrence/progression without evidence of distant metastasis. Median time to local recurrence/progression following surgery or definitive CRT was 13.1 months (IQR, 7.8-17.5 months). Mean diameter of locally recurrent/progressive disease at SBRT was 2.7 cm (SD, 0.9 cm). All patients underwent re-irradiation of the pancreatic bed with SBRT administered over 5 consecutive daily fractions. Sixteen of 18 patients (89%) received a total dose of 25 Gy (5 Gy ×5), while 1 patient received 20 Gy (4 Gy ×5) and 1 patient received 27 Gy (5.5 Gy ×5). Five patients (28%) received additional chemotherapy following SBRT.
Table 1. Demographic, baseline disease, and treatment characteristics.
| Characteristic | Quantitative measure |
|---|---|
| Demographics | |
| Median age [range] | 64 [42-72] |
| Sex | |
| No. female [%] | 8 [44] |
| No. male [%] | 10 [56] |
| Baseline disease | |
| Tumor location within pancreas | |
| No. head [%] | 11 [61] |
| No. body [%] | 3 [17] |
| No. tail [%] | 4 [22] |
| Initial disease stage | |
| No. resectable who underwent curative surgery [%] | 15 [83] |
| No. with positive margins [%] | 6 [40] |
| No. with positive lymph nodes [%] | 11 [73] |
| No. locally advanced who underwent definitive CRT [%] | 3 [17] |
| First course of radiotherapy (conventional fractionation) | |
| No. treated neoadjuvantly [%] | 4 [22] |
| No. treated adjuvantly [%] | 11 [61] |
| No. treated definitively [%] | 3 [17] |
| Median dose in Gy [IQR] | 50.4 [45.0-50.4] |
| Median fraction size in Gy [IQR] | 1.8 [1.8-1.8] |
| No. who received concurrent chemotherapy [%] | 17 [94%] |
| No. 5-fluorouracil-based [%] | 10 [55] |
| No. gemcitabine-based [%] | 7 [39] |
| Second course of radiotherapy (re-irradiation with SBRT) | |
| ECOG performance status prior to SBRT | |
| No. ECOG 0-1 [%] | 17 [94] |
| No. ECOG 2 [%] | 1 [6] |
| No. who received 5 Gy ×5 (total dose of 25 Gy) [%] | 16 [89] |
| No. who received other regimen [%] | 2 [11]a |
| No. of patients requiring treatment break or dose reduction [%] | 0 [0] |
| Systemic therapy | |
| No. who received gemcitabine-based maintenance chemotherapy prior to SBRT [%] | 18 [100] |
| No. who received immunotherapy prior to SBRT [%] | 2 [11]b |
| No. who received chemotherapy following SBRT [%] | 5 [28]c |
| Mean no. of cycles received [SD] | 2.8 [2.4] |
CRT, chemoradiation therapy; SBRT, stereotactic body radiation therapy; ECOG, Eastern Cooperative Oncology Group; Gy, Gray; a1 received 4 Gy ×5; 1 received 5.5 Gy ×5; bboth patients received a pancreatic tumor cell vaccine with ipilimumab; of these 2 patients, one survived for 18.7 months following SBRT and the other currently remains alive 25 months following SBRT; c4 received gemcitabine-based regimens, 1 received a 5-FU-based regimen
Efficacy
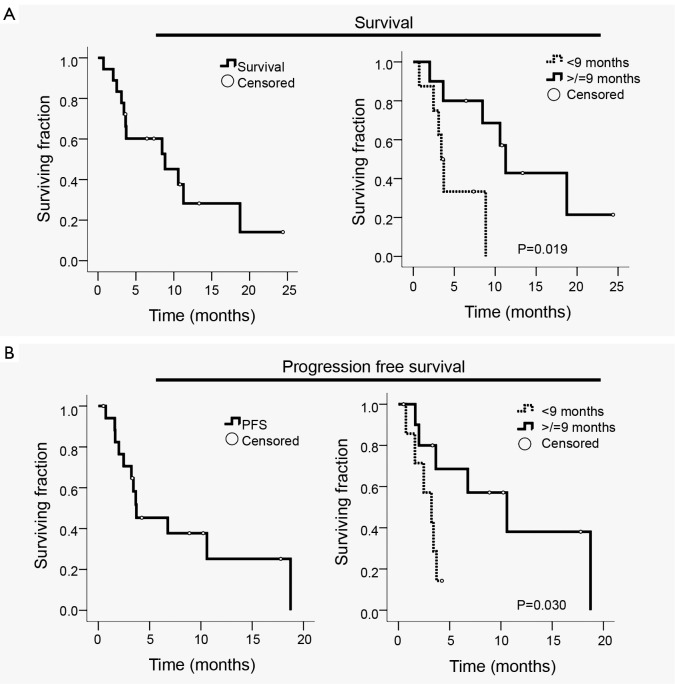
Median follow-up was 34.3 months (range, 6.4-61.6 months) and median interval from local recurrence/progression to SBRT was 2.4 months (IQR, 1.8-5.1 months). Median survival measured from the time of radiographically documented local recurrence/progression was 12.0 months (95% CI, 9.9-14.0 months). Median survival measured from SBRT was 8.8 months (95% CI, 1.2-16.4 months) (Figure 1A). Based on previously published surgical data regarding re-resection for isolated local recurrence (2), patients were dichotomized based on whether local recurrence/progression occurred fewer or greater than 9 months following surgery or definitive CRT. The resulting two groups were similar in regard to age, gender, ECOG performance status, median tumor diameter, and histologic grade as well as rates of margin positivity, lymph node involvement, perineural invasion, and lymphovascular invasion (all P>0.05; Table 2). Patients who recurred/progressed locally within 9 months of surgery or definitive CRT (n=8) survived for a median of only 3.4 months (95% CI, 2.7-4.2 months) after SBRT versus 11.3 months (95% CI, 9.6-12.9 months) for patients who recurred/progressed after more than 9 months (n=10; P=0.019) (Figure 1A).
Figure 1.
Kaplan-Meier plots. A. Survival measured from the date of SBRT initiation for all patients (left panel) and stratified by time to local recurrence/progression after surgery or definitive chemoradiation of <9 or ≥9 months (right panel); B. Progression-free survival (PFS) measured from the date of SBRT initiation for all patients (left panel) and stratified by time to local recurrence/progression after surgery or definitive chemoradiation of <9 or ≥9 months (right panel). Open circles indicate censored patients
Table 2. Comparison of demographic and clinicopathologic characteristics between patients who developed isolated local recurrence/progression less than versus greater than 9 months following surgery or definitive chemoradiation therapy (CRT).
| All patients (n=18) | <9 months (n=8) | >9 months (n=10) | P | |
|---|---|---|---|---|
| Characteristic | ||||
| Age: median years [range] | 64 [42-72] | 60 [45-72] | 67 [42-71] | 0.51 |
| Gender: No. male [%] | 10 [56] | 4 [50] | 6 [60] | 0.67 |
| ECOG: No. 0-1 [%] | 17 [94] | 7 [88] | 10 [100] | 0.25 |
| Tumor diameter: median cm [range] | 3 [2.0-4.5] | 3 [2.5-4.5] | 3 [2.0-4.0] | 0.48 |
| Grade: No. moderately differentiated [%] | 13 [72] | 5 [63] | 8 [80] | 0.41 |
| Resection margin: No. positive [%] | 6 [33] | 3 [38] | 3 [30] | 0.74 |
| No. with lymph node involvement [%] | 11 [61] | 5 [63] | 4 [60] | 0.91 |
| No. with perineural invasion [%] | 10 [56] | 5 [63] | 5 [50] | 0.60 |
| No. with lymphovascular invasion [%] | 10 [56] | 4 [50] | 6 [60] | 0.67 |
| Outcome | ||||
| Median survival (months) | 8.8 | 3.4 | 11.3 | 0.02 |
| Median progression-free survival (months) | 3.7 | 3.2 | 10.6 | 0.03 |
ECOG, Eastern Cooperative Oncology Group
Median progression-free survival (PFS) following SBRT was 3.7 months (95% CI, 0.6-6.9 months) (Figure 1B). Patients who had recurred/progressed more than 9 months following surgery or definitive CRT had a longer median PFS (10.6 months, 95% CI, 3.1-18.0 months) compared with patients who had recurred/progressed within 9 months (3.2 months, 95% CI, 1.3-5.2 months; P=0.030) (Figure 1B). Rates of freedom from local progression at 6 and 12 months were 78% (14 of 18 patients) and 62% (5 of 8 patients), respectively. Of the 12 patients who died during the follow-up period, 8 (67%) remained free from local progression during the interval from SBRT until death. In general, for the patients who did not exhibit local progression, SBRT achieved tumor stabilization, but did not cause a radiographically-evident reduction in tumor size. Seven of the 18 patients (39%) had reported symptoms of abdominal/back pain prior to SBRT; effective symptom palliation was achieved in 4 of these 7 patients (57%) according to follow-up history and physical examination performed within 4-8 weeks of SBRT.
Toxicity
All patients completed SBRT without treatment breaks or dose reductions. Five patients (28%) experienced acute grade 2 toxicity manifesting as fatigue, abdominal pain, anorexia, nausea, and diarrhea. No acute grade ≥3 toxicity was observed. One patient (6%) experienced late toxicity in the form of small bowel obstruction (grade 3). No other late toxicity has been observed at a median follow-up of 8.2 months from SBRT (10.6 months for patients currently alive).
Discussion
Limited treatment options exist for patients with isolated local recurrence/progression of pancreatic cancer after aggressive multimodality therapy including prior irradiation. Select patients are eligible for re-resection, yet locally recurrent disease is often unresectable as a consequence of vascular involvement, post-radiation fibrosis, or poor performance status. In the largest surgical series examining re-resection with curative intent, resection of disease was achieved in only 16 of 30 patients (53%) who underwent re-laparotomy, and, of these, just 6 (38%) had negative margins, while 3 (19%) were R1 and 7 (44%) were R2 (2). Median survival following re-resection was 11.4 months, while in-hospital morbidity and mortality were 20% and 7%, respectively. Laparotomy additionally interrupted systemic therapy for several weeks. In contrast, SBRT in the setting of locally advanced pancreatic cancer has been shown to have a mild toxicity profile, to achieve high rates of local control, and to require 5 days or fewer for delivery with swift resumption of systemic therapy afterwards (19-21,24) while remaining more cost-effective than conventional radiotherapy or chemotherapy alone (25,26).
Authors of the current study have previously made several prospective reports on SBRT as definitive therapy for locally advanced pancreatic adenocarcinoma (19-21,24). These studies delivered 25 Gy in one fraction [biologically equivalent dose (BED) early/late: 87.5/233.3 Gy], which resulted in acute grade 2 and 3 toxicity ranging from 15-21% and 0-11%, respectively. Extended follow-up from these studies demonstrated late grade ≥3 toxicity to occur at a rate of 9%, typically manifesting as duodenal stricture or perforation (22). These rates were closely reproduced at other institutions, which collectively showed acute and late grade ≥3 toxicity rates of 0-8% and 0-9%, respectively (26-29). Our results (0% acute, 6% late grade ≥3 toxicity) closely correspond to these previously published figures, despite the fact that all patients had undergone conventionally fractionated CRT prior to SBRT. One potential implication of our data, therefore, is that re-irradiation with 5-fraction SBRT (median BED early/late: 37.5/66.7 Gy) may be no more toxic than SBRT administered to radiation-naïve patients, though admittedly the less aggressive dosing regimen employed in the current study renders direct comparison of toxicity rates between studies difficult. One prospective (20) and two retrospective studies (26,30) have examined a similar scenario involving administration of a planned SBRT boost shortly following conventional CRT and offer comparable results with acute and late grade ≥3 toxicity ranging from 0-13% and 0-7%, respectively. It is important to note, however, that the limited median survival of patients with pancreatic cancer may hinder accurate assessment of the true rate of late toxicity following SBRT.
The trials examining SBRT discussed above (19-21) demonstrated excellent local control rates (81-100%), but minimal impact on median survival, which was similar to that observed in our study (8.8 months) at 7.6-11.8 months. This is likely explained by the propensity of pancreatic cancer to microscopically disseminate early (31), rendering local salvage therapy ineffective for prolonging survival due to subsequent emergence of occult distant metastases. Notably, however, two patients in our series who received a pancreatic tumor cell vaccine with ipilimumab prior to local recurrence/progression demonstrated extended survival after SBRT. While we cannot confirm the role of SBRT in prolonging survival in these cases, it is possible that these patients manifested an improved immune response to their tumors following SBRT, similar to the abscopal effect recently reported for patients with melanoma (32,33).
In order to prevent administration of futile local therapy, one strategy is to give chemotherapy for 2-6 months and reassess for metastases before administering re-irradiation with SBRT (30). While this selection approach is preferable, some patients with acute local symptoms may require a more rapid decision regarding local therapy. Our data indicate that SBRT is more effective in prolonging survival for patients who develop isolated local recurrence/progression ≥9 months after surgical resection or definitive CRT. Therefore, in patients for whom a 2-6 month course of chemotherapy is not feasible due to acute symptoms or inability to tolerate further systemic therapy, the decision to give salvage SBRT without induction chemotherapy could be based on the interval between surgery or definitive CRT and local recurrence/progression. Those recurring/progressing after a prolonged time interval (≥9 months) would be more likely to benefit from SBRT, while those recurring/progressing within 9 months would be better served by palliative measures directed at symptom relief (e.g., nerve block, stenting, surgical bypass) with or without salvage chemotherapy.
In conclusion, re-irradiation with hypofractionated SBRT appears to be a safe and reasonable option for palliation of isolated local recurrence or progression of pancreatic adenocarcinoma following previous conventional CRT. Conclusions regarding efficacy are strongly limited by the small number of patients, retrospective study design, and patient heterogeneity. However, our study suggests that a group of patients who locally recur or progress greater than 9 months from surgery or definitive CRT may have a better prognosis regarding long-term survival and may therefore benefit most from re-irradiation with SBRT given their higher likelihood of living long enough to experience morbidity from eventual local progression. Given the limited data currently available regarding the use of SBRT for salvage treatment of isolated local recurrence or progression of pancreatic adenocarcinoma following previous radiotherapy, these findings may inform clinical decision making and future trial design for this unique patient population.
Acknowledgements
We thank Joyce Schanne for her incredibly valuable help.
Funding: This work was supported by the generosity of the Claudio X. Gonzalez Family Foundation, the Simkins Family Foundation, the Flannery Family Foundation, the Alexander Family Foundation, the Keeling Family Foundation, the DeSanti Family Foundation, and the McKnight Family Foundation.
Disclosure: The authors declare no conflict of interest.
References
- 1.Thomas RM, Truty MJ, Nogueras-Gonzalez GM, et al. Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. J Gastrointest Surg 2012;16:1696-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleeff J, Reiser C, Hinz U, et al. Surgery for recurrent pancreatic ductal adenocarcinoma Ann Sur 2007;245:566-72. [DOI] [PMC free article] [PubMed]
- 3.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: Postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997;225:621-33; discussion 633-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77 [DOI] [PubMed] [Google Scholar]
- 5.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502 [DOI] [PubMed] [Google Scholar]
- 6.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA 2008;299:1019-26 [DOI] [PubMed] [Google Scholar]
- 7.Hernandez JM, Morton CA, Al-Saadi S, et al. The natural history of resected pancreatic cancer without adjuvant chemotherapy. Am Surg 2010;76:480-5 [PubMed] [Google Scholar]
- 8.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer 1990;66:56-61 [DOI] [PubMed] [Google Scholar]
- 9.Van den Broeck A, Sergeant G, Ectors N, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2009;35:600-4 [DOI] [PubMed] [Google Scholar]
- 10.Asiyanbola B, Gleisner A, Herman JM, et al. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-flurouracil-based chemoradiation therapy: effect of number of metastatic lymph nodes and lymph node ratio. J Gastrointest Surg 2009;13:752-9 [DOI] [PubMed] [Google Scholar]
- 11.Sperti C, Pasquali C, Piccoli A, et al. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg 1997;21:195-200 [DOI] [PubMed] [Google Scholar]
- 12.Sharma C, Eltawil KM, Renfrew PD, et al. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol 2011;17:867-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221:721-31; discussion 731-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogawa K, Shibuya H, Uchida N, et al. Postoperative external beam radiotherapy for resected pancreatic adenocarcinoma: impact of chemotherapy on local control and survival. Anticancer Res 2010;30:2959-67 [PubMed] [Google Scholar]
- 16.Rudra S, Narang AK, Pawlik TM, et al. Evaluation of predictive variables in locally advanced pancreatic adenocarcinoma patients receiving definitive chemoradiation. Pract Radiat Oncol 2012;2:77-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zacharias T, Oussoultzoglou E, Jaeck D, et al. Surgery for recurrence of periampullary malignancies. J Gastrointest Surg 2009;13:760-7 [DOI] [PubMed] [Google Scholar]
- 18.Wilkowski R, Thoma M, Bruns C, et al. Combined chemoradiotherapy for isolated local recurrence after primary resection of pancreatic cancer. JOP 2006;7:34-40 [PubMed] [Google Scholar]
- 19.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;58:1017-21 [DOI] [PubMed] [Google Scholar]
- 20.Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2005;63:320-3 [DOI] [PubMed] [Google Scholar]
- 21.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2008;72:678-86 [DOI] [PubMed] [Google Scholar]
- 22.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009;115:665-72 [DOI] [PubMed] [Google Scholar]
- 23.Didolkar MS, Coleman CW, Brenner MJ, et al. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg 2010;14:1547-59 [DOI] [PubMed] [Google Scholar]
- 24.Wild AT, Chang DT, Goodman KA, et al. A phase 2 multi-institutional study to evaluate gemcitabine and fractionated stereotactic radiotherapy for unresectable, locally advanced pancreatic adenocarcinoma. Pract Radiat Oncol 2013;3:S4-5 [DOI] [PubMed] [Google Scholar]
- 25.Murphy JD, Chang DT, Abelson J, et al. Cost-effectiveness of modern radiotherapy techniques in locally advanced pancreatic cancer. Cancer 2012;118:1119-29 [DOI] [PubMed] [Google Scholar]
- 26.Seo Y, Kim MS, Yoo S, et al. Stereotactic body radiation therapy boost in locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2009;75:1456-61 [DOI] [PubMed] [Google Scholar]
- 27.Rwigema JC, Parikh SD, Heron DE, et al. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol 2011;34:63-9 [DOI] [PubMed] [Google Scholar]
- 28.Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2010;78:735-42 [DOI] [PubMed] [Google Scholar]
- 29.Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011;81:e615-22 [DOI] [PubMed] [Google Scholar]
- 30.Lominska CE, Unger K, Nasr NM, et al. Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas. Radiat Oncol 2012;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haeno H, Gonen M, Davis MB, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 2012;148:362-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med 2012;366:2035; author reply 2035-6 [DOI] [PubMed] [Google Scholar]