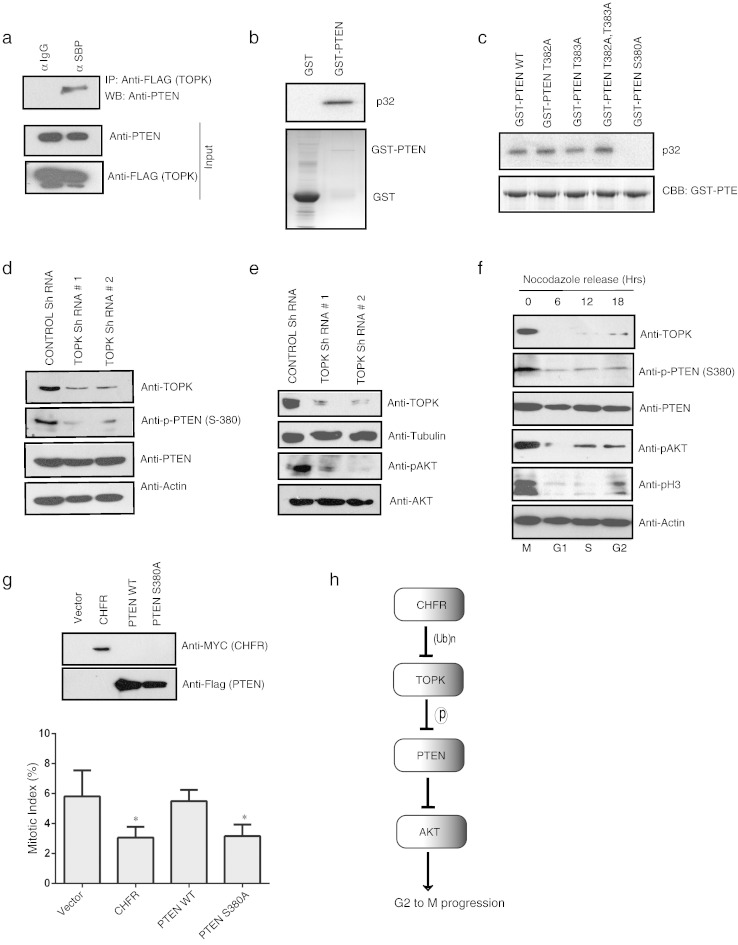
Fig. 4.
TOPK phosphorylates PTEN and is important for mitotic entry. (a) 293 T cell lysate expressing triple tagged TOPK was used for immunoprecipitation by either anti-IgG sepharose or streptavidine sepharose beads and the interaction of PTEN was detected by immunoblotting with anti-PTEN antibody. (b) An in vitro kinase assay was performed on bacterially purified GST or GST–PTEN using a recombinant TOPK holoenzyme in the presence of [γ-p32] ATP. Samples were resolved on 10% SDS-PAGE and PTEN phosphorylation was detected by autoradiography. (c) An in vitro kinase assay was performed on purified wild type PTEN and various PTEN mutants using an active TOPK enzyme in the presence of [γ-p32] ATP and the phosphorylation was shown by autoradiography. (d) PTEN phosphorylation was determined by immunoblotting with phospho-PTEN antibody using HeLa cells transfected with control shRNA or TOPK shRNA containing vectors. (e) Levels of phospho-Akt were determined by immunoblotting with phospho-Akt (serine 473) antibody. (f) HeLa cells synchronized in mitosis by Nocodazole treatment were released into subsequent cell cycle stages. Cell lysates collected at various times were analyzed for the levels of indicated proteins by immunoblotting with their specific antibodies. (g) HeLa cells were transfected as indicated and the expression of the CHFR and PTEN was shown by immunoblotting. Mitotic index is calculated as in Fig. 3b. (h) Proposed model to show the role of TOPK and PTEN in CHFR mediated mitotic checkpoint.