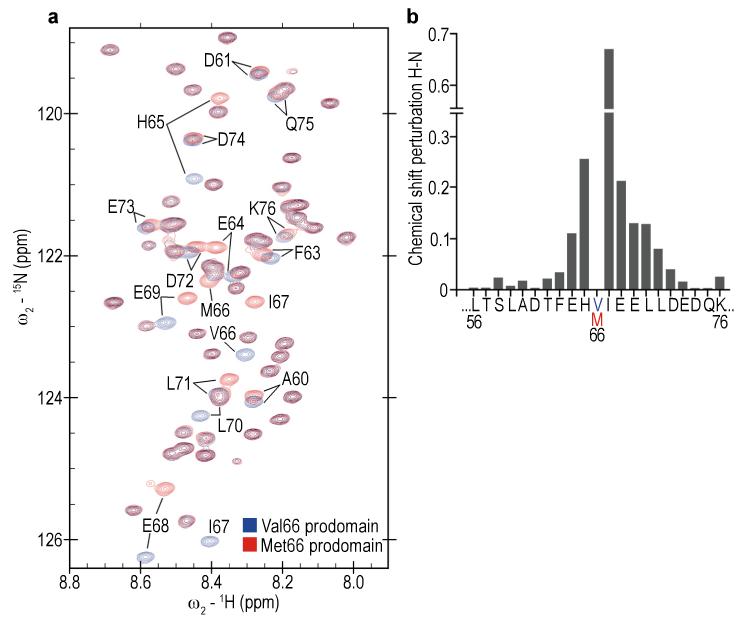
Figure 3.
Impact of the Val66Met substitution on the structure of the BDNF prodomain. (a) Overlay of the heteronuclear single-quantum coherence (HSQC) spectrum on the Val66 (blue) and Met66 (red) prodomains. Each cross-peak (chemical shift) corresponds to one residue within the sequence of the prodomain (one chemical shift for each covalently bonded pair of 1H-15N atoms assigned to specific amides within the prodomain sequence). Full HSQC spectra of Val66 and Met66 prodomains are available in Supplementary Fig. S5. The backbone chemical shifts for the prodomains are deposited in the Biological Magnetic Resonance Bank: Val66 ID number: 19358. Met66 ID number: 19357. (b) Chemical shift deviation (Δδ) between BDNF Val66 and Met66 prodomains showed that changes induced by the substitution are localized to seven residues (E64, H65, I67, E68, E69, L70, L71) neighboring the Val66Met substitution site. The variation in Δδ for residues 23-55, and 77-113 outside the display window was between 0.007 and 0.0037 ppm.