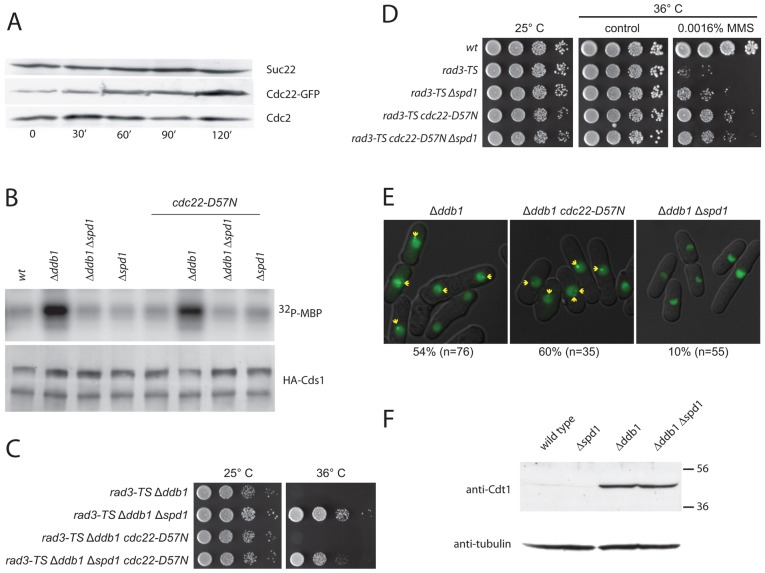
Fig. 1.
cdc22-D57N does not suppress checkpoint activation and meiosis in Δddb1. (A) Cdc22 levels increase upon γ-irradiation. Western blots showing the levels of Suc22, Cdc22–GFP and Cdc2 in exponentially growing cells exposed to ionizing radiation (500 Gy) at time point zero. Samples were collected at the indicated time points (minutes). (B) Cds1 checkpoint kinase activation by excess Spd1 is not suppressed by cdc22-D57N. As marker for DNA-structure-dependent checkpoint activation, Cds1 kinase activity was monitored after immunoprecipitation from the indicated protein extracts by determining its ability to phosphorylate myelin basic protein (32P-MBP), as shown in the upper panel. The lower panel is a western blot for precipitated Cds1. (C) cdc22-D57N does not suppress checkpoint dependency of Δddb1cells. Serial dilutions of the indicated strains were spotted onto solid rich medium and incubated for 3 days at the indicated temperatures before photography. (D) dNTPs are limiting for repair upon rad3ts inactivation at 36°C. Experiments were performed as in C, but included treatment with MMS as indicated. (E) Formation of Ssb1–GFP foci in Δddb1 cells was suppressed by Δspd1 but not by cdc22-D57N. The Ssb1–GFP fusion protein was expressed from the nmt41 promoter in exponentially growing cells of indicated genotypes and detected by fluorescent microscopy. Yellow arrows point to distinct foci. The percentage of cells with at least one nuclear focus is indicated below the micrographs. (F) Western blot showing Cdt1 and tubulin content in the indicated strains.