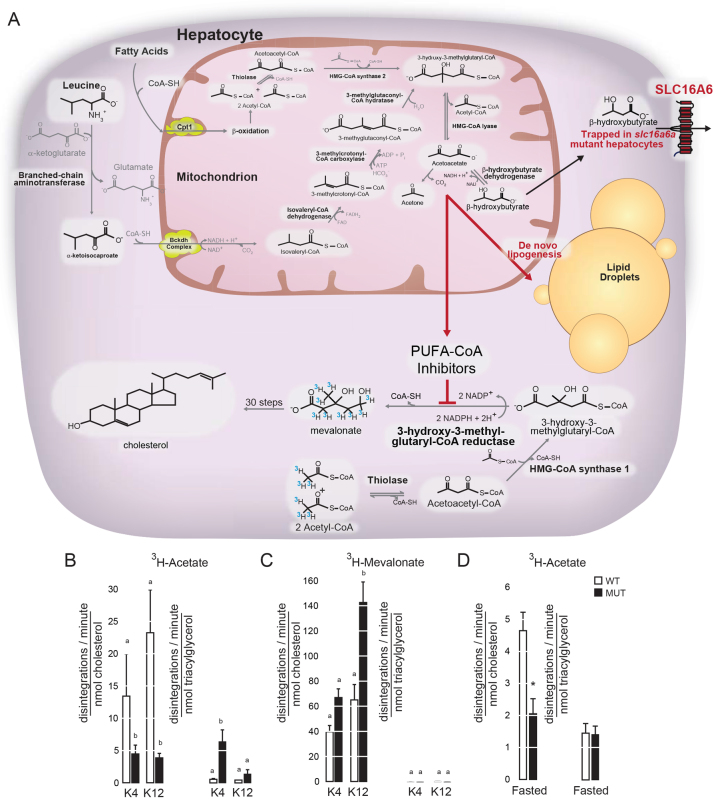
Fig. 4.
In vivo inhibition of Hmgcr in slc16a6a mutant livers. (A) Ketogenesis involves a series of reactions mostly in the mitochondria of hepatocytes, where fatty-acid-derived and select ketogenic-amino-acid-derived carbon atoms are oxidized to acetoacetate. In this scheme, catabolism of acetate (from fatty acids) and the branched-chain amino acid leucine are shown in detail. The terminal product of this pathway is acetoacetate, which can undergo spontaneous decarboxylation to acetone, or be partially reduced to β-hydroxybutyrate. When ketone body export is prevented by mutation of zebrafish slc16a6a, the trapped ketone body carbon atoms are diverted to de novo lipogenesis, but not to cholesterol. Cholesterol biosynthesis is prevented by inhibition of Hmgcr by the PUFACoAs that accumulate during de novo lipogenesis. The 3H-marked isotopes injected intraperitoneally were used to address whether there was a block in Hmgcr activity in vivo. Note that Hmgcs2 is lacking in zebrafish, eliminating this pathway of acetate consumption. (B,C) Wild-type (WT) and slc16a6a mutant (MUT) animals fed ketogenic diets for 45 days were injected intraperitoneally with 3H-labeled acetate and 3H-labeled mevalonate, and the incorporation of radiotracers into cholesterol and triacylglycerol was measured. Results are reported as disintegrations per minute per nanomole of the indicated lipid. Groups with different letters are statistically different, P≤0.05, Duncan’s multiple range test. All data mean ± s.e.m. (n=3 per group). (D) Incorporation of radiolabeled acetate into cholesterol and triacylglycerol in WT and MUT animals subjected to a 10-day fast. Groups with different letters are statistically different, P≤0.001, two-sided Student’s t-test. All data mean ± s.d. (n=5 per group).