Abstract
Tembusu virus (TMUV; Ntaya serocomplex) was detected in two pools of mosquitoes captured near Sangkhlaburi, Thailand, as well as from sera from sentinel ducks from the same area. Although TMUV has been isolated from several mosquito species in Asia, no studies have ever shown competent vectors for this virus. Therefore, we allowed mosquitoes captured near Sangkhlaburi to feed on young chickens that had been infected with TMUV. These mosquitoes were tested approximately 2 weeks later to determine infection, dissemination, and transmission rates. Culex vishnui developed high viral titers after feeding on TMUV-infected chicks and readily transmitted virus to naïve chickens. In contrast, Cx. fuscocephala seemed less susceptible to infection, and more importantly, zero of five fuscocephala with a disseminated infection transmitted virus by bite, indicating a salivary gland barrier. These results provide evidence for the involvement of Culex mosquitoes in the transmission of TMUV in the environment.
Introduction
In support of efforts to develop rapid nucleic acid-based diagnostic techniques for the detection of arthropod-borne pathogens, mosquitoes were collected in rice paddy farming villages throughout Kamphaeng Phet, Thailand. The field site was selected for the high incidence rate of dengue (DEN) cases, and all four serotypes of DEN virus (DENV) are endemic to the area.1 A second field site located near Kong Mong Tha-Sangkhlaburi (Kanchanaburi province), Thailand, was selected for the high probability for the collection of Japanese encephalitis virus (JEV)-infected Culex spp. mosquitoes. This location was also the site of a serological study (acute and convalescent sera from humans and sera from sentinel animal) conducted by the Armed Forces Research Institute of Medical Sciences (AFRIMS), Bangkok, Thailand, in early 2002 to determine the cause of fevers of unknown origins in humans.
Previous vector surveillance studies conducted in Kamphaeng Phet in 1982 using Centers for Disease Control and Prevention (CDC) light traps identified 35 isolates of JEV, 18 isolates of Tembusu virus (TMUV), three untyped flaviviruses, three alphaviruses, and four unidentified viruses from 345,173 mosquitoes.2 The TMUV isolates were from pools of Cx. vishnui, Cx. tritaeniorynchus, and Cx. gelidus. In 1992, TMUV was isolated from Cx. tritaeniorynchus collected in Chiang Mai, Thailand.3 TMUV is a positive-sense single-stranded RNA virus belonging to the Ntaya virus serogroup of the Flaviviridae family,4 and it was first isolated in Malaysia in 1955 from Cx. tritaeniorynchus (http://wwwn.cdc.gov/arbocat/catalog-listing.asp?VirusID=470). TMUV was also isolated from Cx. vishnui and Cx. vishnui subgroup mosquitoes in Malaysia in 1970 and then again in 1974.5 Since that time, neutralizing antibodies to TMUV as well as other flaviviruses have been detected in humans sera collected in Sarawak from 1962 to 1966, Indonesia in 1977, and Borneo from 1996 to 1997.6–8 Although antibody responses have been measured in human sera, disease attributed to this virus has not been documented in humans and may be obscured by diseases caused by DENV and JEV. However, disease (ovarian hemorrhage and hyperemia with neurological sequelae) in animals has been noted in China and Malaysia.9–11 Here, we report the isolation of TMUV in Thailand and provide the first report of vector competence testing to show that field-caught mosquitoes can be efficient vectors for spreading the virus in Thailand and elsewhere.
Materials and Methods
Field site location and mosquito collections.
During February of 2002, mosquitoes were collected from rice paddy farming villages in the vicinity of Kamphaeng Phet (an agrarian area of 8,608 km2 located approximately 360 km northwest of Bangkok) and Kong Mong Tha-Sangkhlaburi (an agrarian area of 19,483 km2 located approximately 280 km west of Bangkok and 18 km from the border of Myanmar), Thailand, using animal-baited traps (primarily using pigs at Kamphaeng Phet and cows at Kong Mong Tha-Sangkhlaburi) or light traps (American Biophysics Corp., North Kingstown, RI) supplemented with carbon dioxide (dry ice). The daily arthropod collections from the light traps and the backpack-aspirated animal traps were taken to the field laboratory for processing. Mosquitoes were killed by freezing, identified to species, pooled into groups of ∼25 females, and tested for the presence of viral RNA by reverse transcription polymerase chain reaction (RT-PCR) as previously described12 and using MA/cFD2 flavivirus primers.4 The remaining mosquito homogenate was transported on dry ice back to the US Army Medical Research Institute of Infectious Diseases (USAMRIID), Fort Detrick, MD, for additional testing and genetic sequencing. Also, live field-caught mosquitoes from Kamphaeng Phet, Thailand, from 2005 were transported back to USAMRIID, where they were provided an uninfected blood meal, and the F1 progeny was used for vector competence testing.
Virus and virus assays.
TMUV (strains Thai-MLO305 and Thai-JSL385) -positive mosquito homogenates that were detected in the field were returned to the laboratory and passed in C6/36 cells grown in Hank's minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum, 100 units penicillin, and 100 μg streptomycin per 1 mL and 0.075% NaHCO3 at 28°C in a humidified incubator. Cell culture supernatants containing virus were clarified using 0.20-μm surfactant-free cellulose acetate filters (Fisher Scientific, Inc., Pittsburg, PA). Tembusu strain Thai-MLO305, containing approximately 104 plaque-forming units (PFU) per milliliter (as determined by plaque assay on Vero cell monolayer), was used to inoculate the different vectors and hosts that were used in the vector competence studies presented here. Cell culture supernatants from sentinel animal sera passed in C6/36 cells were tested by RT-PCR for the presence of flavivirus RNA, and the resulting data concerning TMUV are present here. The use of sentinel animals was part of an unrelated serological study conducted during January and February of 2002, and it was designed to determine the etiological agents causing cases of human febrile illnesses of unknown origin in Sangkhlaburi, Thailand. Data concerning the serosurvey and sentinel animals are not part of this study and will not be presented here.
Viremia profile studies.
TMUV viremia profiles were determined using young leghorn chickens (Gallus gallus); 1- to 5-day-old chickens were inoculated subcutaneously with 0.1 mL diluted cell culture supernatant containing approximately 103 PFU TMUV strain Thai-MLO305. These chickens were bled daily from the jugular vein (0.1 mL blood into 0.9 mL heparinized diluents and 10% heat-inactivated fetal bovine serum in Medium 199 with Earle's salts [Invitrogen, Inc., Carlsbad, CA], NaHCO3, and antibiotics), and the blood suspensions were frozen at −70°C until tested for virus by plaque assay.
Vector competence studies.
Cx. vishnui and Cx. fuscocephala mosquitoes from Thailand were allowed to feed on 2- to 4-day-old leghorn chicks that had been inoculated with 103 PFU TMUV strain Thai-MLO305 1–3 days earlier. Immediately after mosquito feeding, 0.1 mL blood were obtained from the jugular vein of each chicken and handled as describe above to determine the viremia at the time of mosquito feeding. After exposure to the viremic chickens, engorged mosquitoes were transferred to 3.8-L screen-topped cardboard cages held at 26°C with a 16:8-hour (light:dark) photoperiod. After an incubation period of 15 to 18 days, the mosquitoes were allowed to refeed on 1- to 2-day-old chickens either individually or in groups of three to five mosquitoes to determine if they could transmit virus by bite. Immediately after the transmission attempt, the mosquitoes were killed by freezing and identified to species. The feeding status was determined, and their legs and bodies were triturated separately in 1 mL diluent. Infection was determined by recovery of virus from the mosquito tissue suspension. If virus was recovered from its body but not its legs, the mosquito was considered to have a non-disseminated infection limited to its midgut. In contrast, if virus was recovered from both the body and leg suspensions, the mosquito was considered to have a disseminated infection.13 We defined the infection and dissemination rates as the percentages of mosquitoes tested that contained virus in their body and legs, respectively. Chickens used in the transmission attempts were bled from the jugular vein 2 days after mosquito feeding, and the blood was handled as described above. Recovery of virus from this blood indicated transmission.
To more efficiently examine virus transmission, unfed mosquitoes from Thailand were inoculated intrathoracically14 with 0.3 μL virus preparation containing 104.2 PFU TMUV/mL (100.7 PFU/mosquito) and held for 9 days before being allowed to feed on 1- to 2-day-old chicks. Mosquitoes and blood specimens from these chicks were processed as described for the orally exposed mosquitoes. We used the modified Wald method of calculating 95% confidence intervals (95% CIs; http://www.measuringusability.com/wald.htm).15
Laboratory sequencing and phylogenetic analysis.
RT-PCR amplification products (approximately one-half of the PCR reaction; about 15 μL) from positive mosquito pools were purified using a Qiaquick PCR Purification Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer's instructions. Automated sequencing was performed using an ABI 310 genetic analyzer with a Big-Dye 1.0 Sequencing Kit (PE Biosystems, Inc., Foster City, CA) according to the manufacturer's instructions. Primer, excess nucleotides, and buffer were removed from the Big-Dye sequencing reaction by eluting the material from a Sephadex G-50 (GE Healthcare Biosciences, Inc., Piscataway, NJ) column equilibrated with water. Sequences were aligned using the MegaAlign program (Lasergene analysis software; DNASTAR, Inc., Madison, WI), and sequence ends were trimmed to a uniform length. Phylogenetic analysis was conducted according to the work by Kondig and others16 using the Clustal W program of Megalign to calculate bootstrap values with a default setting of 1,000 trials (iterations) and a seed value of 111.
Results
Isolation of TMUV from field-collected mosquitoes and duck sera.
During May of 2002, 2,994 female mosquitoes were captured in traps set around Kamphaeng Phet and sorted to species in the field. Of 183 pools that were tested for the presence of flavivirus RNA, 1 pool of Cx. vishnui tested positive for JEV, and 2 pools of Cx. vishnui (N = 2,326 Cx. vishnui/118 pools) tested positive for flavivirus and negative for JEV and DENV. They were identified as TMUV (see below). From the Kong Mong Tha-Sangkhlaburi field site, 2,229 female mosquitoes were collected and sorted by species into 258 pools. No flavivirus RNA was detected in these mosquito pools; however, only 90 Cx. vishnui/seven pools were collected at this site. Also, lower numbers of Cx. tritaeniorhynchus (the principle vector of JEV) were collected from these sites: 354 mosquitoes/20 pools for Kamphaeng Phet and 481 mosquitoes/30 pools for Kong Mong Tha-Sangkhlaburi compared with historical collections.2 The minimum infection rate (MIR) for TMUV-infected Cx. vishnui was calculated as 0.9. Additionally, three TMUV isolates were identified during a subsequent visit to Kamphaeng Phet, Thailand, in March of 2008 from 442 pools (11,009 mosquitoes; MIR = 0.3) of Cx. tritaeniorynchus mosquitoes (unpublished data) compared with no isolations from Cx. vishnui (69 pools and 1,641 mosquitoes).
During the serological study in January and February of 2002 in Sangkhlaburi, Thailand, serum samples from sentinel animals (ducks, cows, pigs, and chickens) were collected, passed in C6/36 cells, and assayed for the presence of different viruses. Seven serum samples collected from ducks that were then passed three to four times in C6/36 cells were identified as containing an unknown virus. We tested these cell culture supernatants by RT-PCR; we found that four cell culture supernatants contained TMUV RNA and that three cell culture supernatants contained JEV RNA. No viruses were detected in C6/36 cell culture-passed cow, pig, or chicken sera.
Laboratory sequencing and phylogenetic analysis.
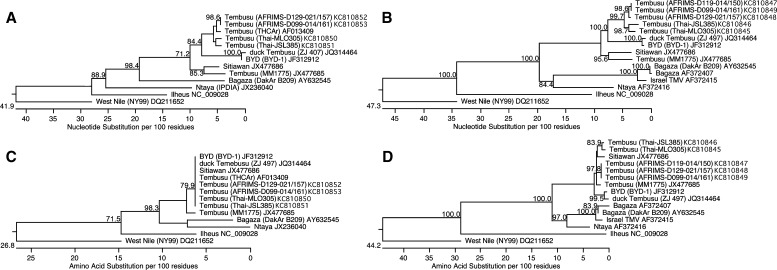
To verify the identity of the flavivirus found in the mosquitoes, the mosquito homogenates were passaged in C6/36 cells. After 3–5 days, viral RNA was easily detected by RT-PCR in filtered supernatants, and supernatant applied to Vero cell monolayers produced plaques in a standard plaque assay. Sequencing a 160-bp portion of the ns5 gene from the two TMUV isolates, Thai-MLO305 and Thai-JSL385, showed a 97.5% nucleotide identity between them and a 91.2% and 90.6% nucleotide identity, respectively, with TMUV strain THCAr (Figure 1A). However, all three viruses encoded identical amino acid sequences corresponding to the respective ns5 gene fragment (Figure 1C). An additional comparison of an 866-bp segment of the envelope gene revealed a nucleotide identity of 97.1% between the two mosquito isolates and a nucleotide identity of 89.2% and 89.0%, respectively, with TMUV strain MM1775 (Figure 1B). For this envelope fragment, the mosquito isolates' amino acid sequences were 99.0% identical and 97.2% and 97.6% identical to both Sitiawan virus and TMUV strain MM1775, respectively (Figure 1D). Both mosquito isolates were also similarly related to the duck TMUV strain BYD-1 at 91.2% for the ns5 gene fragment (100% amino acid identity) and 89.9% for the envelope gene segment (95.5% amino acid identity with strain Thai-MLO305) (Figure 1).
Figure 1.
Phylogenetic analysis of TMUV isolated from mosquitoes and duck sera from Thailand in May of 2002. (A) Nucleotide or (C) amino acid analysis of a 160-bp fragment from the ns5 gene. (B) Nucleotide or (D) amino acid analysis of an 866-bp segment from the envelope gene. The virus name is followed by the strain name in parentheses, if known, and the GenBank accession number. Bootstrap values ≥ 70 are shown at the nodes.
Of seven sentinel duck serum samples from western Thailand that were passaged in C6/36 cells, four tested positive for TMUV RNA using RT-PCR. Laboratory sequence analysis confirmed the presence of the virus in the cell culture-passed sera. Phylogenetic analysis of TMUV strain AFRIMS-D129-014/157 and TMUV strain AFRIMS-D099-014/161 showed a nucleotide identity of 100% for the ns5 gene fragment and a nucleotide identity of 99.5% for the envelope gene segment (100% amino acid identity) between the two isolates (Figure 1). Comparison of TMUV strain AFRIMS-D099-014/161 with the mosquito isolate TMUV strain Thai-MLO305 revealed a 97.5% nucleotide identity with the ns5 gene fragment and a 97.0% nucleotide identity with the envelope gene segment compared with 90.0% and 90.7% nucleotide identities, respectively, with TMUV strain MM1775. As with the mosquito isolates, the duck serum isolates were similarly identical to the duck TMUV strain BYD-1 at 90.0% for the ns5 gene fragment (100% amino acid identity) and 90.6% for the envelope gene segment (96.5% amino acid identity with strain AFRIMS-D099-014/161) (Figure 1).
Overall, the phylogenetic analysis showed that the mosquito TMUV isolates grouped with the duck serum TMUV isolates and a historical isolate from Thailand and that this grouping was distinct from historical TMUV isolates from Malaysia and the newly isolated duck TMUV from China (Figure 1).
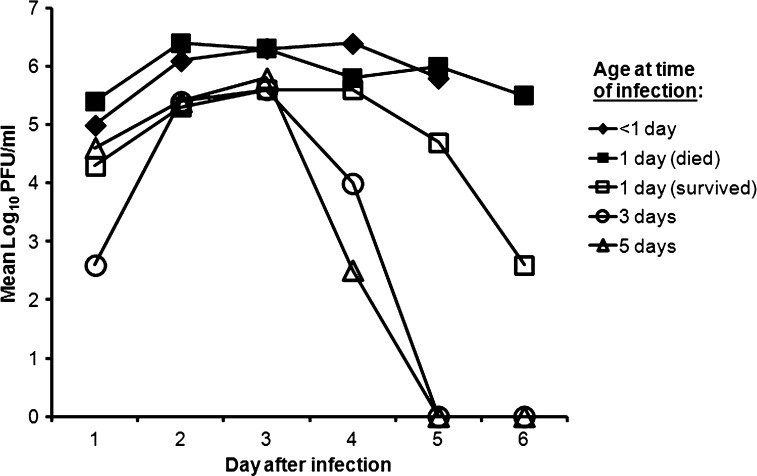
Figure 2.
Replication of TMUV in chickens by age at the time of inoculation.
Viremia profile studies and vector competence results.
The chickens that were less than 1 day old when inoculated with TMUV (strain Thai-MLO305) developed a peak viremia with a mean titer of 106.4 PFU/mL on day 4 after inoculation, and all died by day 6. Chickens that were 1 day old when inoculated with virus developed one of two disease courses. About one-half of these chickens developed a peak viremia of about 106.4 PFU/mL and died by day 7, whereas one-half of these chickens developed a peak viremia of 105.6 PFU/mL and survived the infection. Chickens inoculated when 3 or 5 days old produced a similar pattern to 1-day-old chickens that survived with a peak mean viremia of about 105.5 PFU/mL, and all survived the infection.
Viremia in the chicks used to expose mosquitoes to TMUV ranged from 104.5 to 107.6 PFU/mL blood. Nearly all of the specimens tested were Cx. vishnui, which were highly susceptible to infection with TMUV at all of the doses tested, with 94% of all of the specimens becoming infected if fed on a chicken with a viremia ≥ 105.5 PFU/mL (Table 1). Not only were infection rates high but at least 84% of the infected mosquitoes had a disseminated infection at each of the exposure doses. Every chicken fed on by either a Cx. vishnui with a disseminated infection (n = 1 single mosquito, two pools of two mosquitoes, and one pool of five mosquitoes) after oral exposure or a Cx. vishnui that had been inoculated with TMUV (N = 4) was infected with TMUV. Therefore, Cx. vishnui was not only highly susceptible to infection, but it also readily transmitted TMUV. In contrast, Cx. fuscocephala did not seem to be a competent vector of TMUV. Although sample sizes were very small, zero of one (0%) and one of two (50%) of those chickens that were fed upon with a viremia of 104.5–105.6 PFU/mL, respectively, became infected. Also, none of five Cx. fuscocephala that had been inoculated with TMUV transmitted virus when fed on susceptible chickens, indicating the presence of a salivary gland barrier.17
Table 1.
Infection and dissemination rates for Cx. vishnui after feeding on chickens infected with TMUV strain Thai-MLO305
| Viremia (log10 PFU/mL) | No. tested | Infection rate* | Dissemination rate† | Dissemination (I) rate‡ |
|---|---|---|---|---|
| 104.5±0.1 | 29 | 76 (55–88) | 66 (47–80) | 86 (66–96) |
| 105.5±0.3 | 33 | 94 (79–99) | 79 (62–90) | 84 (67–93) |
| 10≥6.1 | 46 | 98 (88–99) | 93 (82–98) | 96 (84–99) |
Infection rate is the percentage of mosquitoes containing virus in their bodies (95% CI).
Dissemination rate is the percentage of mosquitoes, regardless of their infection status, containing virus in their legs (95% CI).
Dissemination (I) rate is the percentage of infected mosquitoes containing virus in their legs (95% CI).
Discussion
This work is the first demonstration of the ability of TMUV to replicate in and be transmitted by a mosquito. In nature, TMUV has been associated primarily with members of the Cx. vishnui subgroup.2,3 This group includes Cx. vishnui and Cx. tritaeniorhynchus, which are morphologically very similar and both considered important vectors of JEV.18 We also evaluated the potential of Cx. fuscocephala to transmit TMUV. All three mosquito species like to remain outdoors and breed in groundwater (puddles, rice paddies, ponds, and ditches); they prefer to feed on cows and then on pigs, but they will feed on humans, wild birds, and poultry (domestic chickens, turkeys, and ducks) based on host abundance.5,18–22 Although Cx. vishnui prefers to feed on cattle, they will feed on birds to a greater extent than will Cx. tritaeniorynchus.5 Our observations are consistent with the report by Leake and others2 detailing the identification of TMUV in Thailand in Cx. tritaeniorynchus (MIR = 0.04), Cx. vishnui (MIR = 0.2), and Cx. gelidus (MIR = 0.1). Taken together, the data presented here extend the role of Cx. vishnui in the sylvatic and urban transmission cycles involving TMUV and wild and domestic avian hosts in Thailand and support the previous observation that TMUV uses birds as hosts and Cx. vishnui subgroup in their transmission cycle in Malaysia.5 Because both Cx. vishnui and Cx. vishnui subgroups prefer to feed on large animals and not humans, this finding may explain why very few individuals have seroconverted to this virus.5–8 Although TMUV may not be currently causing a recognized disease in humans, the possibility exists that this virus may emerge as a human pathogen.23 This lack of recognition may be because of illnesses caused by infection with TMUV being attributed to either DENV or JEV, two other flaviviruses found in the same area, and it is complicated by the cross-reactivity of serologic testing among these flaviviruses. This risk that TMUV might cause human illness is enhanced, because it has been reported to cause encephalitis in non-human primates.24
Despite TMUV not being recognized as causing disease in humans (or poultry in Thailand, although TMUV was isolated from ducks from Thailand), TMUV has had a significant impact on the duck industry in China (first recognized in April of 2010), with reports of an approximately 90% drop in egg production and 5–30% mortality in the birds.9,11,25 Phylogenetic analysis of the TMUV strains isolated from the Thai ducks and mosquitoes indicates that they are closely related to the duck TMUV from China and may be causing an unrecognized morbidity and mortality in Thailand in wild birds and poultry, because infection of leghorn chicks with TMUV strain Thai-MLO305 caused growth retardation and death in young chickens. This effect was also noted previously for Sitiawan virus (a strain of TMUV, although it was originally named differently) and its consequence on broiler chicks.10 The data presented here also support the role of mosquitoes in the spread of duck TMUV in China in addition to the possible oral route of spread as described for sparrows in China.21 Although TMUV has not emerged as a recognized disease in humans or animals in Thailand, continuous surveillance will be necessary to prevent economic losses caused by the emergence of a more virulent TMUV strain.
ACKNOWLEDGMENTS
The authors thank David J. Dohm and SGT Michael Delgado for the assistance in maintaining the mosquito colonies and Jim Pecor (Biosystematics Unit, Department of Entomology, Walter Reed Army Institute of Research) for identifying mosquitoes. We also thank Paul Gibbs for statistical support and Kathy Kenyon for critically reading the manuscript. Research was conducted under an Institutional Animal Care and Use Committee (IACUC)-approved protocol in compliance with the Animal Welfare Act, PHS Public Health Service (PHS) Policy, and other federal statutes and regulations relating to animals and experiments involving animals. The facility where this research was conducted is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011.
Disclaimer: The mention of trade names or commercial products does not constitute endorsement or recommendation for use by the Department of the Army or the Department of Defense. The opinions and assertions contained herein are the opinions and assertions of the authors, and they are not to be construed as official or reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Financial support: This work was supported through the joint partnership between the US Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, and the Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand, and it was funded in part by the Military Infectious Disease Research Program Projects U0014_02_RD and U0014_03_RD.
Authors' addresses: Monica L. O'Guinn, Military Infectious Diseases Research Program, US Army Medical Research and Materiel Command, Fort Detrick, Frederick, MD, E-mail: monica.oguinn@us.army.mil. Michael J. Turell, Virology Division, US Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, E-mail: michael.j.turell@us.army.mil. Ampornpan Kengluecha, Boonsong Jaichapor, and James W. Jones, Department of Entomology, US Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand, E-mails: ampornpank@afrims.org, boonsongj@afrims.org, and james.jones@afrims.org. Prasan Kankaew, Department of Geography, State University of New York, Buffalo, NY, E-mail: prasankankaew@yahoo.com. R. Scott Miller and Russell E. Coleman, US Army Medical Materiel Development Activity, Frederick, MD, E-mails: robert.s.miller@us.army.mil and russell.coleman@us.army.mil. Timothy P. Endy, Department of Medicine, Upstate Medical University, State University of New York, Syracuse, NY, E-mail: endyt@upstate.edu. John S. Lee, Division of Entomology, Walter Reed Army Institute of Research, Silver Spring, MD, E-mail: john.s.lee13@gmail.com.
Reprint requests: John S. Lee, Division of Entomology, Walter Reed Army Institute of Research, 503 Robert Grant Avenue, Silver Spring, MD 20910, E-mail: john.s.lee13@gmail.com.
References
- 1.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 2.Leake CJ, Ussery MA, Nisalak A, Hoke CH, Andre RG, Burke DS. Virus isolations from mosquitoes collected during the 1982 Japanese encephalitis epidemic in northern Thailand. Trans R Soc Trop Med Hyg. 1986;80:831–837. doi: 10.1016/0035-9203(86)90397-4. [DOI] [PubMed] [Google Scholar]
- 3.Pandey BD, Karabatsos N, Cropp B, Tagaki M, Tsuda Y, Ichinose A, Igarashi A. Identification of a flavivirus isolated from mosquitos in Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health. 1999;30:161–165. [PubMed] [Google Scholar]
- 4.Kuno G. Universal diagnostic RT-PCR protocol for arboviruses. J Virol Methods. 1998;72:27–41. doi: 10.1016/s0166-0934(98)00003-2. [DOI] [PubMed] [Google Scholar]
- 5.Wallace HG, Rudnick A, Rajagopal V. Activity of Tembusu and Umbre viruses in a Malaysian community: mosquito studies. Mosq News. 1977;37:35–42. [Google Scholar]
- 6.Gordon Smith CE, Simpson DIH, Peto S, Bowen ETW, McMahon D, Platt GS, Way H, Bright WF, Maidment B. Arbovirus infections in Sarawak: serological studies in man. Trans R Soc Trop Med Hyg. 1974;68:96–104. [Google Scholar]
- 7.Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ, Cropp BC, Andau M, Spielman A, Gubler DJ. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001;64:310–316. doi: 10.4269/ajtmh.2001.64.310. [DOI] [PubMed] [Google Scholar]
- 8.Olson JG, Ksiazek TG, Gubler DJ, Lubis SI, Simanjuntak G, Lee VH, Nalim S, Juslis K, See R. A survey for arboviral antibodies in sera of humans and animals in Lombok, Republic of Indonesia. Ann Trop Med Parasitol. 1983;77:131–137. doi: 10.1080/00034983.1983.11811687. [DOI] [PubMed] [Google Scholar]
- 9.Yan P, Zhao Y, Zhang X, Xu D, Dai X, Teng Q, Yan L, Zhou J, Ji X, Zhang S, Liu G, Zhou Y, Kawaoka Y, Tong G, Li Z. An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology. 2011;417:1–8. doi: 10.1016/j.virol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Kono Y, Tsukamoto K, Abd Hamid M, Darus A, Lian TC, Sam LS, Yok CN, Di KB, Lim KT, Yamaguchi S, Narita M. Encephalitis and retarded growth of chicks caused by Sitiawan virus, a new isolate belonging to the genus Flavivirus. Am J Trop Med Hyg. 2000;63:94–101. doi: 10.4269/ajtmh.2000.63.94. [DOI] [PubMed] [Google Scholar]
- 11.Su J, Li S, Hu X, Yu X, Wang Y, Liu P, Lu X, Zhang G, Liu D, Li X, Su W, Lu H, Mok NS, Wang P, Wang M, Tian K, Gao GF. Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One. 2011;6:e18106. doi: 10.1371/journal.pone.0018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Guinn ML, Lee JS, Kondig JP, Fernandez R, Carbajal F. Field detection of eastern equine encephalitis virus in the Amazon Basin region of Peru using reverse transcription-polymerase chain reaction adapted for field identification of arthropod-borne pathogens. Am J Trop Med Hyg. 2004;70:164–171. [PubMed] [Google Scholar]
- 13.Turell MJ, Rossignol PA, Spielman A, Rossi CA, Bailey CL. Enhanced arboviral transmission by mosquitoes that concurrently ingested microfilariae. Science. 1984;225:1039–1041. doi: 10.1126/science.6474165. [DOI] [PubMed] [Google Scholar]
- 14.Rosen L, Gubler D. The use of mosquitoes to detect and propagate dengue viruses. Am J Trop Med Hyg. 1974;23:1153–1160. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- 15.Agresti A, Coull BA. Approximate is better than “Exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–126. [Google Scholar]
- 16.Kondig JP, Turell MJ, Lee JS, O'Guinn ML, Wasieloski LP., Jr Genetic analysis of South American eastern equine encephalomyelitis viruses isolated from mosquitoes collected in the Amazon Basin region of Peru. Am J Trop Med Hyg. 2007;76:408–416. [PubMed] [Google Scholar]
- 17.Kramer LD, Hardy JL, Presser SB, Houk EJ. Dissemination barriers for western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am J Trop Med Hyg. 1981;30:190–197. doi: 10.4269/ajtmh.1981.30.190. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Tuno N, Yen NT, Nam VS, Takagi M. Influence of the distribution of host species on adult abundance of Japanese encephalitis vectors Culex vishnui subgroup and Culex gelidus in a rice-cultivating village in northern Vietnam. Am J Trop Med Hyg. 2008;78:159–168. [PubMed] [Google Scholar]
- 19.Mitchell CJ, Chen PS, Boreham PF. Host-feeding patterns and behaviour of 4 Culex species in an endemic area of Japanese encephalitis. Bull World Health Organ. 1973;49:293–299. [PMC free article] [PubMed] [Google Scholar]
- 20.Arunachalam N, Samuel PP, Hiriyan J, Rajendran R, Dash AP. Short report: observations on the multiple feeding behavior of Culex tritaeniorhynchus (Diptera: culicidae), the vector of Japanese encephalitis in Kerala in southern India. Am J Trop Med Hyg. 2005;72:198–200. [PubMed] [Google Scholar]
- 21.Tang Y, Diao Y, Yu C, Gao X, Ju X, Xue C, Liu X, Ge P, Qu J, Zhang D. Characterization of a Tembusu virus isolated from naturally infected House Sparrows (Passer domesticus) in northern China. Transbound Emerg Dis. 2013;60:152–158. doi: 10.1111/j.1865-1682.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 22.Lien JC. Non-anopheline mosquitoes of Taiwan: annotated catalog and bibliography. Pac Insects. 1962;4:615–649. [Google Scholar]
- 23.Mackenzie JS, Williams DT. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health. 2009;56:338–356. doi: 10.1111/j.1863-2378.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 24.Simpson DI, Smith CE, Bowen ET, Platt GS, Way H, McMahon D, Bright WF, Hill MN, Mahadevan S, Macdonald WW. Arbovirus infections in Sarawk: virus isolations from mosquitoes. Ann Trop Med Parasitol. 1970;64:137–151. doi: 10.1080/00034983.1970.11686675. [DOI] [PubMed] [Google Scholar]
- 25.Cao Z, Zhang C, Liu Y, Ye W, Han J, Ma G, Zhang D, Xu F, Gao X, Tang Y, Shi S, Wan C, He B, Yang M, Lu X, Huang Y, Diao Y, Ma X. Tembusu virus in ducks, china. Emerg Infect Dis. 2011;17:1873–1875. doi: 10.3201/eid1710.101890. [DOI] [PMC free article] [PubMed] [Google Scholar]