Figure 11.
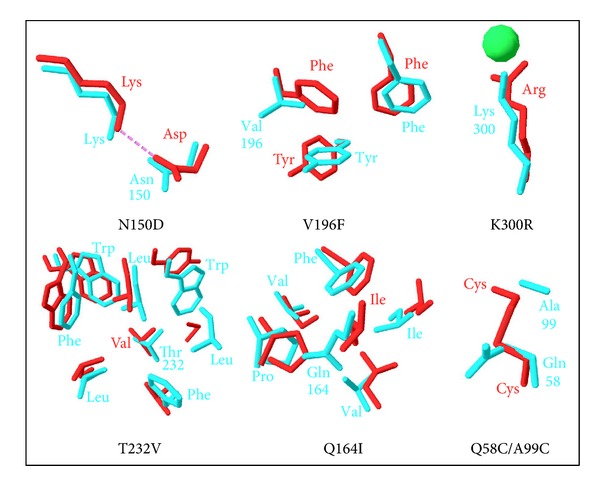
Amino acid substitutions in a psychrophilic alpha-amylase (blue) in comparison with the structure of its mesophilic homologue (red). The substitution N150D disrupts a salt bridge with the corresponding Lys side chain in the cold-adapted protein. The substitution V196F disrupts a triple face-to-edge aromatic interaction. Substitution K300R results in a monodentate coordination of the chloride ion, instead of a bidentate coordination by Arg in the mesophilic protein, as demonstrated by the crystal structure of the single mutant [272]. Substitutions T232V and Q164I decrease the apolarity of hydrophobic core clusters in the psychrophilic enzyme and the double substitution Q58C/A99C eliminates a disulfide bond. Adapted from [114].
