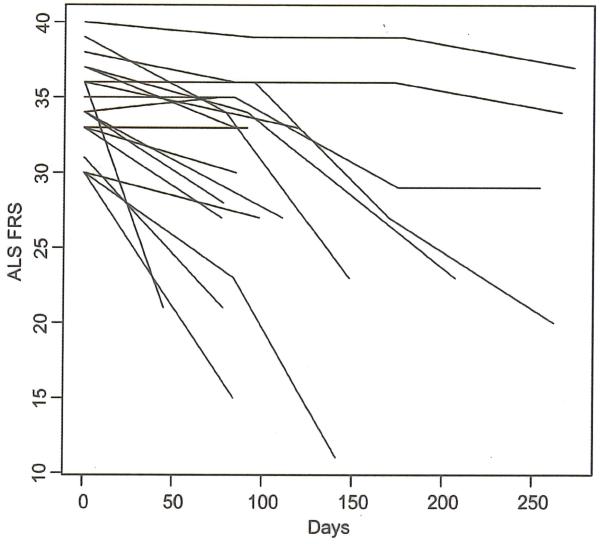
Figure 1.
ALSFRS over time for each of the 19 subjects in the study that had at least one post-baseline measurement for this parameter.
| Month | Mean changes from baseline |
P (t-test) |
p (Signed Wil- coxon) |
|---|---|---|---|
| 3 | −4.7 (95% CI: −6.9, −2.5) |
0.0003 | 0.0006 |
| 6 | −9.6 (95% CI: −16.0, −3.1) |
0.011 | 0.016 |
| 9 | −7.3 (95% CI: −16.7, +2.2) |
0.093 | 0.125 |
| In topiramate controls: Month Mean changes from baseline |
|||
| 3 | −2.2±0.2 | ||
| 6 | −5.0±0.5 | ||
| 9 | −6.5±0.5 | ||