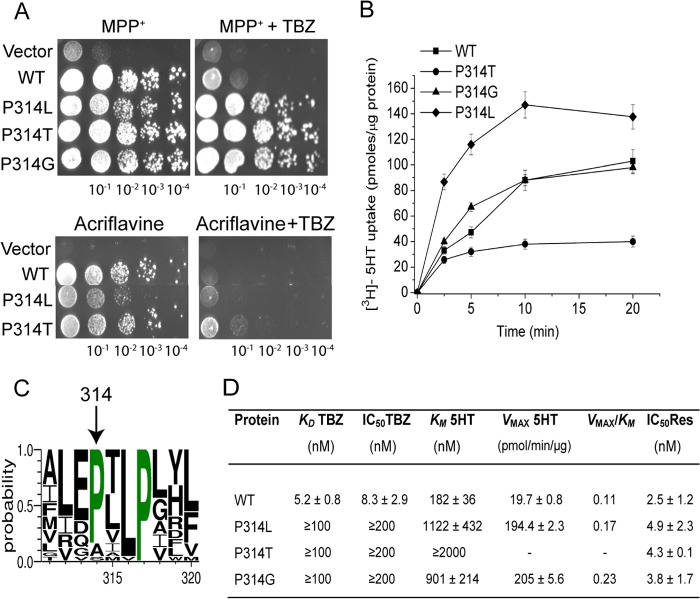
FIGURE 4.
The highly conserved Pro314 is crucial for TBZ binding and plays a role in the transport function of rVMAT2. A, replacement of Pro314 by Leu or Thr confers resistance to TBZ in yeast cells. ADU1–7 cells were transformed with pAES426 (empty vector) or pAES426 harboring rVMAT2 and Pro314 mutants. Growth was assayed and analyzed as described in the legend to Fig. 1A. B, time course of [3H]serotonin transport by Pro314 mutants reconstituted in proteoliposomes. The uptake assay was performed as described in the legend to Fig. 1B. All data are mean ± S.E. of 2–3 experiments. C, conservation of Pro314. The sequence of rVMAT2 was used to query the National Center for Biotechnology Information (NCBI) using the psi-BLAST tool as provided by the NCBI server with default parameters. Multiple sequence alignment and the conservation logo were created as described in the legend to Fig. 1C. Only a fraction of the alignment corresponding to residues 311–320 is shown. D, kinetic properties of Pro314 mutants. Proteoliposomes were prepared from HEK293 cells expressing rVMAT2 or Pro314 mutants. KD for [3H]TBZOH binding, Km, Vmax for [3H]serotonin uptake, and IC50 for reserpine and TBZ, were determined as described in the legend to Fig. 1D.