FIGURE 3.
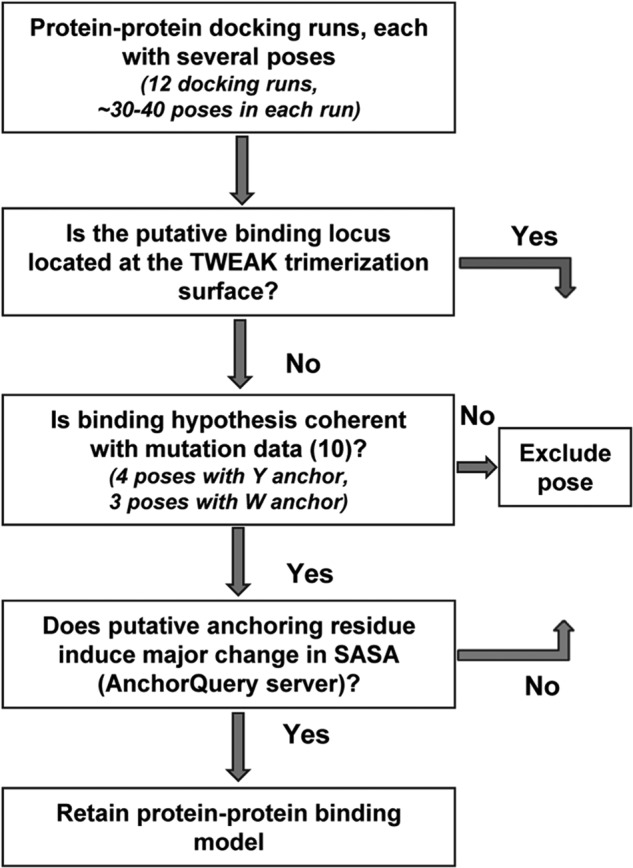
Data-driven decision-making workflow for prioritization of protein-protein docking results. Protein-protein docking of six TWEAK models was performed with two Fn14 models, leading to 12 protein-protein docking runs and hundreds of poses. Poses where the putative binding locus is located at the TWEAK trimerization interface were not considered as valid. Poses with the mutational validated residues Asp45, Lys48, Met50, and Asp62 present at the binding interface were retained. Finally, the AnchorQuery server was used to rank-order the anchoring residues, and those models with Phe, Tyr, or Trp resulting in solvent-accessible surface area loss upon complex formation were retained.
