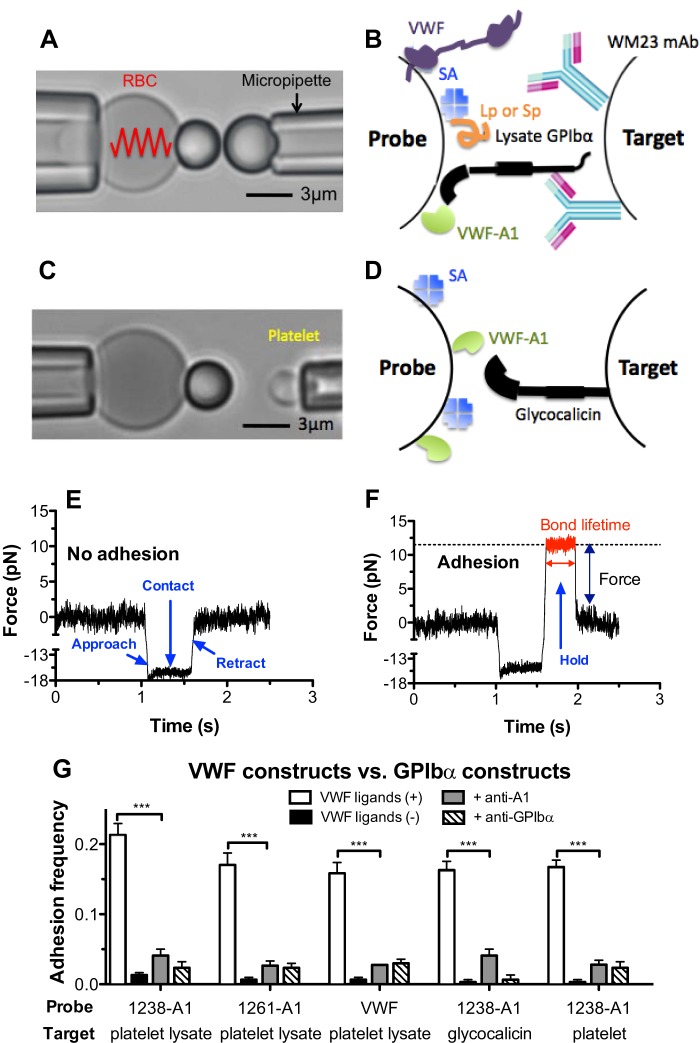
FIGURE 1.
BFP force clamp assay. A and C, BFP photomicrograph. A micropipette-aspirated RBC with a bead (left, termed “probe”) attached to the apex formed a pico-force sensor, as depicted by a spring. It was aligned with another bead (A) or a platelet (C) (right, termed “target”) aspirated by an apposing micropipette. B and D, BFP functionalization with purified molecules. A1 or full-length VWF was covalently coupled to the probe (left). GPIbα was captured from lysates of platelets or CHOαb9 cells by mAb WM23, which was covalently precoated on the target (B, right). Glycocalicin was covalently coupled to the target (D, right). Streptavidin was also covalently coupled to the probe for attachment to biotinylated RBC, as well as coupling the polypeptide Gln1238–Glu1260 (Lp) or scramble polypeptide (Sp). E and F, force versus time traces from two representative test cycles. A target was driven to approach a probe (<1.1 s, ∼0 pN), contacted for 0.5 s (1.1–1.6 s, ∼15 pN), retracted, and ended the cycle if no adhesion was detected (>1.6 s, ∼0 pN) (E) or held at a preset force until dissociation if adhesion was detected (1.6–2 s, ∼12 pN, indicated in red), after which the target was retracted to the starting position (>2 s, ∼0 pN) (F). Lifetime was measured from the point when the clamped force (12 pN) was reached (1.26 s) to the point when the bond dissociated (2 s), signified by a force drop to zero. G, binding specificity. Adhesion frequencies between targets coated with platelet lysate GPIbα, glycocalicin, or platelet GPIbα and probes coated without (−, black columns) or with (+, white columns) 1238-A1 or VWF, in the absence or presence of 50 μg/ml anti-A1 (5D2, gray columns) or anti-GPIbα (AK2, hatched columns) blocking mAb. Each probe-target pair was tested repeatedly for 100 approach-contact-retract cycles to estimate an adhesion frequency. Five probe-target pairs were tested to obtain mean ± S.E. ***, p < 0.001 as assessed by unpaired, two-tailed Student's t test.