FIGURE 6.
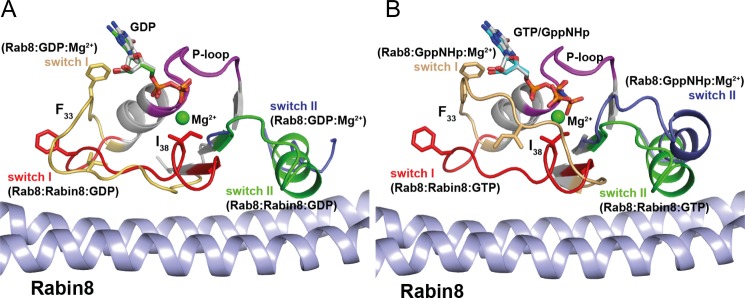
Conformational changes in Rab8·GDP (A) or Rab·GTP (B) upon binding of Rabin8. Superposition of Rab8·GDP/GppNHp and Rab8·Rabin8·GDP/GTP revealed that upon Rabin8 binding, the switch domains adopt highly ordered structures. The driving forces for nucleotide release are as follows. 1) The interaction of Rabin8 sterically occludes the magnesium-binding site by Ile-38 of switch I. The repositioned Ile-38 residue sterically interferes with Mg2+ binding. The release of the Mg2+ ion leads to a reduction in the affinity of the nucleotide for Rab8. 2) Upon Rabin8 binding, Phe-33 undergoes a conformational change. The repositioning of this conserved residue in switch I, which interacts with the guanine base, is part of the driving force in reducing the affinity for the nucleotide.
