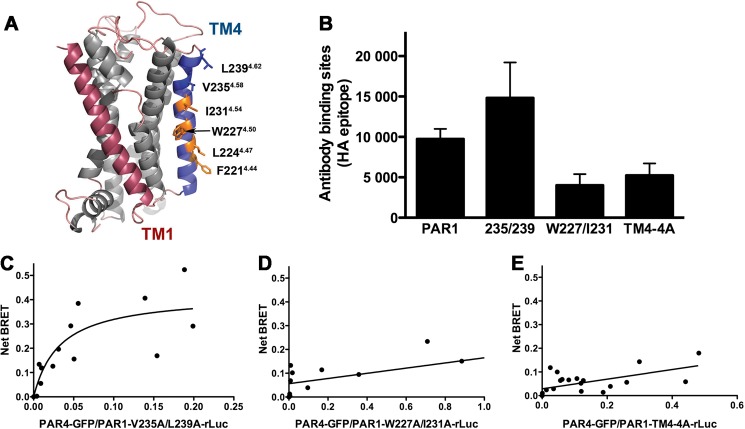
FIGURE 5.
Residues in transmembrane helix 4 of PAR1 mediate interaction with PAR4. A, structural model of PAR1 based on rhodopsin generated with Swiss Modeler. TM1 is indicated in red, and TM4 is in blue. Residues that were analyzed in this study are shown as sticks. The residues in which alanine substitution disrupted α-thrombin-induced heterodimerization are shown in orange. The nomenclature is that of Ballesteros and Weinstein (27). B, the expression of PAR1 wild type and mutants in HEK293 was analyzed by flow cytometry with an anti-HA antibody. C–E, BRET assays were performed in HEK293 cells expressing V5-PAR4-GFP (0-.24 μg) and HA-PAR1-V235A/L239A-rLuc (C), HA-PAR1-W227A/I231A-rLuc (D), or HA-PAR1-TM4-4A-rLuc (0.5 μg) (E). Forty-eight hours post-transfection the cells were treated with α-thrombin (10 nm) for 10 min and analyzed for GFP expression, luciferase activity, and BRET. The data are from two or three independent experiments in which all points were analyzed by global fit to hyperbolic or linear curve.