FIGURE 2.
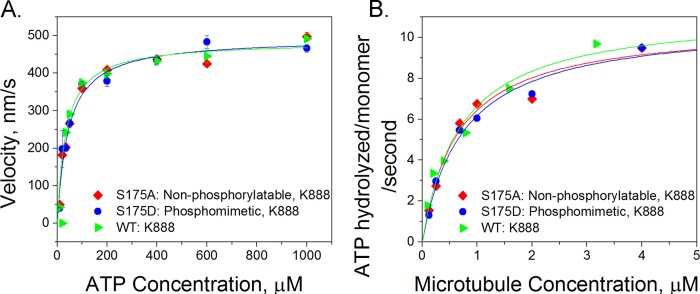
Ser-175 modification does not alter the chemical kinetics of kinesin. Measurements of the affinity of the wild-type K888 (green triangles), S175A (red diamonds), and S175D (blue circles) mutants for ATP and microtubules are shown. A, velocity versus ATP concentration. We measured the velocity of quantum dot cargos moved by single kinesin motors and found no significant difference in the velocity of the three different kinesin mutants as a function of ATP concentration. The error bars shown at each point represent the S.E. velocity at each ATP concentration. The velocities of at least 40 quantum dots were measured and averaged for each data point. B, microtubule-stimulated ATPase activity in which the concentrations of kinesin and ATP were constant but the amount of microtubules in bulk solution was titrated in an enzyme-linked phosphatase assay. For each curve, two or three concentration points were picked, and the assay was repeated to ensure the consistency of the results.
