Fig. 2.
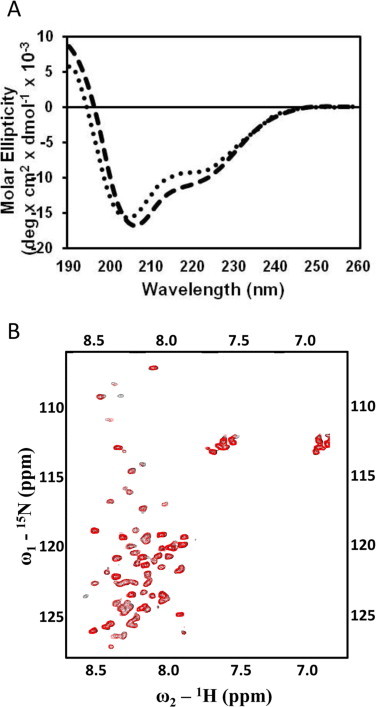
The W(−1)A/P75A double mutant does not undergo complete Ca2+-induced conformational change. (A) The CD spectra of the wild-type ScaADoc (dashed line) and W(−1)A/P75A (dotted line) proteins showcase how complete abrogation of the clasp interaction interferes with the folding of the molecule in the presence of Ca2+. (B) Overlay of the 2D 1H–15N HSQC spectra of the Ca2+-bound (red) and apo-form (black) of the double mutant, W(−1)A/P75A-ScaADoc, wherein the divalent metal cation has little effect on its tertiary structure. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
